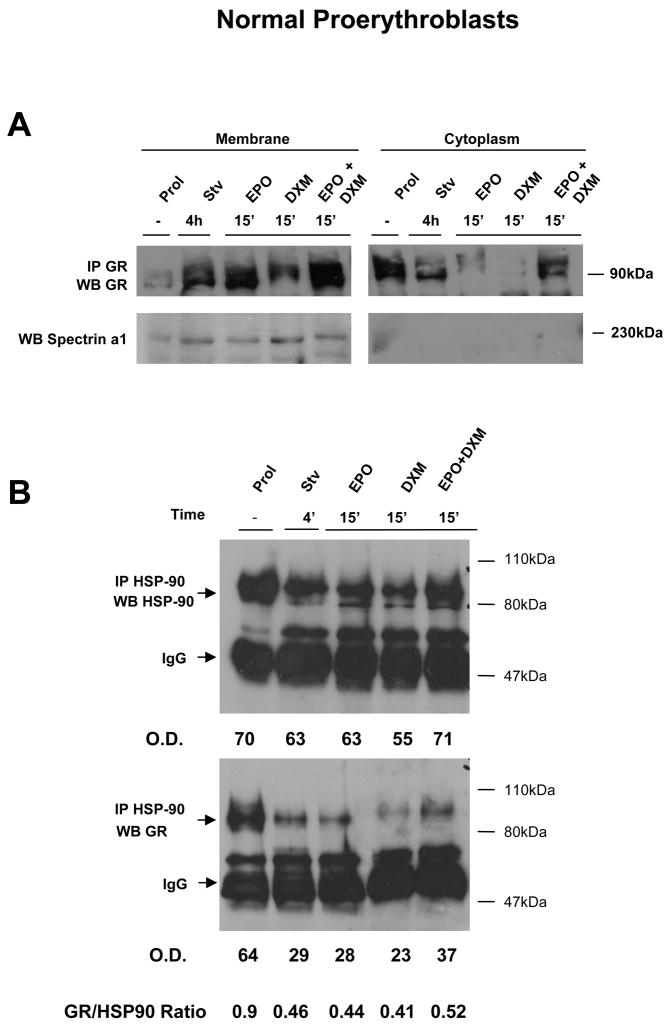
Figure 4. GF starvation, and stimulation with EPO and DXM alone, induces HSP-90-independent translocation of GR from the cytosol to the cell membranes of normal proerythroblasts.
A) Localization of GR in normal proerythroblasts by cell fractionation experiments. Equivalent amount of proteins (30 μg) obtained from the membrane and the cytosol fraction of human proerythroblasts were immunoprecipitated with antibodies specific for GR, separated by SDS-PAGE and probed again by western blot with an antibody specific for GR. The cells were obtained either from HEMA culture (Prol) or GF starved for 4h (Stv) and then treated either with EPO, DXM or the combination of both, as indicated. The amount of spectrin α1 present in the membrane and cytosolic fractions was analyzed by western blot. The relative distribution of GR in the membrane and cytosol fraction, as corrected for the total amount of proteins obtained in the two fractions, of cells exposed to different stimuli is presented in Table 1.
B) Western blot analyses from HSP-90 and GR of whole cell extracts (50 μg) immunoprecipitated with an anti-HSP-90 antibody from human proerythroblasts obtained in HEMA (Prol), GF starved for 4 h (Stv), and then treated for 15′ with EPO (3 U/mL), DXM (10−6 M), or the combination of both, as indicated. The position of the molecular weight markers (in kDa) and of the bands of the expected size for HSP-90, GR and IgG are indicated. The relative intensity of the HSP-90 and GR bands observed in the different lanes was determined by spectrometry, expressed as optical density (OD) and used to calculate the ratio between the two bands.