Abstract
Liver fibrosis is currently assessed by liver biopsy, a costly and rather cumbersome procedure that is unsuitable for frequent patient monitoring, which drives research into biomarkers for this purpose. To investigate whether the serum N-glycome contains information suitable for this goal, we developed a 96-well plate-based serum N-glycomics sample preparation protocol that only involves fluid transfer steps and incubations in a PCR thermocycler yielding 8-aminopyrene-1,3,6-trisulfonic acid-labeled N-glycans. These N-glycans are then ready for analysis on the capillary electrophoresis-based DNA sequencers that are the current standard in clinical genetics laboratories worldwide. Subsequently we performed a multicenter, blinded study of 376 consecutive chronic hepatitis C virus patients for which liver biopsies and extensive serum biochemistry data were available. Among patients, the METAVIR fibrosis stage distribution was as follows: 10.6% F0, 44.4% F1, 20.5% F2, 18.4% F3, and 6.1% F4. We found that the ratio of two N-glycans, here called GlycoFibroTest, correlates with the histological fibrosis stage equally well as FibroTest (ρ = 0.4–0.5 in F1–F4), which is used in the clinic today. Finally using affinity chromatography we depleted sera of immunoglobulin G, and this resulted in a complete removal of the undergalactosylated biantennary glycans from the N-glycome, which are partially determining GlycoFibroTest.
Liver fibrosis in chronic hepatitis patients is currently assessed by liver biopsy. However, this expensive technique can be accompanied by severe complications (1), sampling error (2), and up to 20% interlaboratory variance (3). These shortcomings make it unsuitable for regular monitoring of the patient's condition. Nevertheless monitoring the progression of fibrosis would allow determination of the long term responses to existing therapies (e.g. interferon/ribavirin) and emerging candidates for therapy (e.g. small interfering RNA against heat shock protein 47 (4)). Moreover it is important to follow up patients who have clinically significant fibrosis (METAVIR F2–F4 (5)) and to detect cirrhosis in an early stage because cirrhosis substantially increases the risk of developing hepatocellular carcinoma (HCC)1 (6). Therefore, non-invasive markers are needed as alternatives or at least complements to the liver biopsy golden standard for monitoring fibrosis evolution over time.
Increased levels of extracellular matrix components are useful for monitoring evolution of liver fibrosis, and in this context serum hyaluronate has been thoroughly studied. This linear polysaccharide is a constituent of the extracellular matrix of peripheral tissues (7). Its concentration in the serum increases as liver fibrosis progresses because of an increase in its synthesis by activated hepatic stellate cells and in later fibrosis stages also because of reduced clearance by the liver sinusoidal endothelial cells (8, 9). In patients with clinically significant fibrosis, α2-macroglobulin concentrations also increase (10). This acute phase protein inhibits matrix metalloproteinases, and its production by hepatocytes and activated stellate cells is up-regulated during fibrosis. The aspartate transaminase to platelets ratio index (APRI) (11) measures two routinely assessed parameters: aspartate transaminase concentration and platelet count. Thrombocytopenia during fibrogenesis in patients infected with hepatitis C virus (HCV) can be attributed to hypersplenism (12) as well as to reduced production of thrombopoietin by hepatocytes (13). The increase of serum aspartate transaminase concentration during fibrosis progression may be due to reduced clearance by the liver (14). APRI can predict significant fibrosis and cirrhosis (11).
In an attempt to improve upon these biochemical parameters, several complex regression and classification algorithms have been designed. Tissue inhibitor of metalloproteinases-1, α2-macroglobulin, and hyaluronate are the three components of the FibroSpect test (15) (Prometheus Laboratories, San Diego, CA). Tissue inhibitor of metalloproteinases-1 is produced in the liver mainly by stellate cells and acts as a specific inhibitor of matrix metalloproteinases. Its concentration is increased in advanced liver fibrosis. FibroSpect components are measured in laboratories of the commercial supplier, which should ensure appropriate quality control and reliability. FibroSpect has been validated in HCV patients by some groups (16, 17). FibroTest (18) (Biopredictive, Paris, France) is a binary logistic regression model designed to distinguish between chronic HCV patients who have clinically significant fibrosis (F2–F4) and those who do not (F0–F1). It consists of five serum biochemical markers (α2-macroglobulin, apolipoprotein A-I, γ-glutamyl transpeptidase, haptoglobin, and total bilirubin) as well as the patient's age and gender. The investigators who developed and commercialized the FibroTest algorithm have published several studies to validate it. However, only a few other groups have independently assessed the performance of FibroTest, and few studies compared the performance of the algorithm with the performance of the individual parameters constituting the model and with other fibrosis correlates. Moreover as FibroTest is in principle accessible only via a Web interface in which one enters the clinical chemistry values measured in their own laboratory, it may be quite difficult to assure the quality of output. Indeed measured α2-macroglobulin and apolipoprotein A-I values can be different when measured on two different analyzers even when they have been calibrated against the same standard (Beckman-Coulter, Krefeld, Germany versus Dade Behring, Eschborn, Germany) (19). This is an important issue given the strong dependence of FibroTest on its α2-macroglobulin component (see below). FibroTest results have to be interpreted cautiously because measuring five components not only increases overall variance but also broadens the range of possible interferences (2), such as inflammation (increase in either haptoglobin or α2-macroglobulin), Gilbert syndrome (increase in unconjugated bilirubin), and a decrease in haptoglobin and/or elevation of unconjugated bilirubin because of hemolysis.
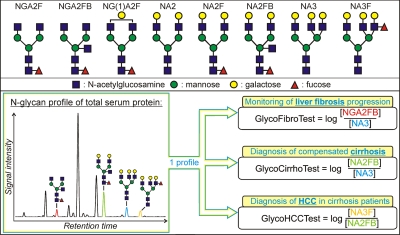
Several new approaches have recently been developed to assess liver fibrosis. FibroScan (20) (Echosens, Paris, France) uses transient elastography to measure the stiffness of the liver, which correlates with the amount of scar tissue formed. Briefly when an elastic shear wave is introduced in the liver by low frequency vibrations, its velocity will depend on the stiffness of the tissue. Although the test is rapid, reproducible, and useful in diagnosing advanced fibrosis (F3–F4), it is rather expensive, and measurements are difficult to perform in obese patients. Increased values are also seen in patients with acute liver inflammation. In 2001 (21) we introduced an ultrasensitive method to profile protein-linked N-glycans using a slab gel-based DNA sequencer. We used this method to identify GlycoCirrhoTest as a serum N-glycome-derived biomarker for advanced liver disease (cirrhosis) (22). However, the method was still rather cumbersome and hard to use. Here we introduce a more simple assay (Fig. 1) that is optimized to prepare 8-aminopyrene-1,3,6-trisulfonic acid (APTS)-labeled N-glycans from total serum protein. All reaction steps can be done in a 96-well plate format only requiring a thermocycler and reagent transfer steps making it suitable for clinical implementation. These samples can then be analyzed on state-of-the-art and high throughput DNA sequencers without changing the settings needed for genomics (23). From a simple serum N-glycome analysis on standard DNA analyzers, three biomarkers can be derived (Fig. 2). GlycoCirrhoTest (22) can distinguish compensated (early stage) cirrhosis from non-cirrhotic chronic liver disease with 79% sensitivity and 86% specificity (both 100% in decompensated cirrhosis). GlycoHCCTest helps in distinguishing cirrhosis patients with HCC from those without (24). Moreover we found indications that GlycoFibroTest, which is the ratio between the agalacto glycans shown in Fig. 2 (NGA2FB) and the fully galactosylated triantennary glycans (NA3; Fig. 2), appeared to rise gradually with increasing fibrosis stage. Consequently this ratio might be a promising marker for the follow-up of liver fibrosis progression. To validate this GlycoFibroTest, we undertook the current study.
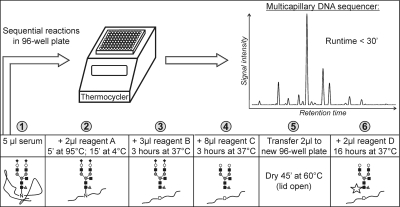
Fig. 1.
Serum N-glycomics sample preparation protocol. APTS-labeled N-glycans are prepared with consecutive reactions (1–6) that are performed in a 96-well plate incubated in a thermocycler (see “Experimental Procedures”). Reagent A, 5% SDS in 10 mm NH4HCO3, pH 8.3; reagent B, 0.5 IUBMB milliunits of peptide N-glycosidase F in 10 mm NH4HCO3, pH 8.3; reagent C, 2 milliunits of α(2→3,6,8,9)-sialidase in 50 mm NH4Ac, pH 5; and reagent D, 1:1 mixture (v/v) of 20 mm APTS in 1.2 m citric acid and 1 m NaCNBH3 in dimethyl sulfoxide. Finally samples are electrokinetically injected in a DNA sequencer using the standard setting for genomics. Analytes migrate toward the anode and are separated based on their size to charge ratio. Black rhombus, sialic acid; white circle, galactose; black square, N-acetylglucosamine; gray circle, mannose; gray triangle, fucose. ′, minutes.
Fig. 2.
Structure and nomenclature of the N-glycans mentioned in this study (upper panel) and N-glycome-derived biomarkers for chronic liver disease (lower panel). From the N-glycan profile derived from total serum protein (N-glycome), one can derive three glycomics-based biomarkers for fibrosis, cirrhosis, and HCC by taking the log-transformed ratio of different glycan species.
EXPERIMENTAL PROCEDURES
Patients—
Initially 400 consecutive chronic HCV patients from the Fibropaca study (25) were enlisted. All participants signed informed consent and did not have any sign of decompensated cirrhosis according to Child-Pugh classification (ascites, encephalopathy, serum total bilirubin >2 mg/dl, serum albumin <3.5 g/dl, and International Normalized Ratio >1.7). Liver biopsies and biochemical parameters were obtained on the same day and analyzed in each of the five participating centers in France. Of these, 376 patients (supplemental table) had a complete data set for GlycoFibroTest, hyaluronate, and FibroTest (and thus its components) and were included in this study. GlycoFibroTest and FibroTest were determined without knowing biopsy results. According to the criteria of Regev et al. (26), 53% of the patients had a biopsy of good quality, 39% had a biopsy of fair quality, and 8% had a biopsy of poor quality. The prevalence of each METAVIR fibrosis stage (5) was 10.6% F0, 44.4% F1, 20.5% F2, 18.4% F3, and 6.1% F4.
N-Glycome Analysis—
Two microliters of 10 mm NH4HCO3, pH 8.3, containing 5% SDS was added to 5 μl of serum (Fig. 1). The samples were then incubated in a PCR thermocycler (5 min at 95 °C followed by 15 min at 4 °C). Subsequently 0.5 IUBMB milliunits of peptide N-glycosidase F (New England Biolabs, Ipswich, MA) in 3 μl of 10 mm NH4HCO3, pH 8.3, containing 3.33% Nonidet P-40 was added, and the reaction mixture was incubated for 3 h at 37 °C. Then 2 milliunits of α(2→3,6,8,9)-sialidase from Arthrobacter ureafaciens (Glyko, San Leandro, CA) in 8 μl of 50 mm NH4Ac, pH 5, was added to the samples followed by incubation for 3 h at 37 °C. From the sample 2 μl was transferred to a new PCR tube or 96-well plate containing 5 μl of Milli-Q water and evaporated to dryness, leaving the tubes and lid of the cycler open (45 min at 60 °C). The glycans were labeled at the reducing terminus by adding 2 μl of a 1:1 mixture (v/v) of 20 mm APTS (Molecular Probes, Eugene, OR) in 1.2 m citric acid and 1 m NaCNBH3 in dimethyl sulfoxide. The samples were incubated at 37 °C for 16 h. The reaction was quenched by adding 200 μl of Milli-Q water, and 10 μl of a 1:800 dilution was analyzed by DNA sequencer-assisted fluorophore-assisted carbohydrate electrophoresis (DSA-FACE) using an ABI 3130 multicapillary sequencer (Applied Biosystems, Foster City, CA) as described earlier by our group (23). The 12 most intense peaks that were detected in all samples (together they accounted for >90% of total serum N-glycans) were quantified using the GeneMapper software of the sequencer. Their peak height intensities were normalized to the total intensity of the measured peaks.
Protein Quantification—
Albumin, IgG, IgA, transferrin, α2-macroglobulin, apolipoprotein A-I, and haptoglobin were measured by immunonephelometry using commercially available antibodies on a Nephelometer II analyzer (both from Dade Behring) (27). A Hitachi 917 Analyzer (Roche Diagnostics, Basel, Switzerland) was used to measure γ-glutamyl transpeptidase, alanine transaminase, and total bilirubin with commercially available reagents. Total protein concentrations of the various fractions obtained after depleting both IgG and albumin were measured on a Modular P system (Roche Diagnostics) by the sensitive pyrogallol red assay (28).
IgG/Albumin Depletion of Sera—
Serum samples from 15 additional chronic HCV patients (three per METAVIR fibrosis stage) were obtained from an outpatient clinic (Ghent University Hospital). Anti-HCV antibodies and HCV RNA were detectable in every patient, and the available sera volumes were high enough to perform the purification experiments. All patients gave their informed consent, and Ghent University Hospital's Ethics Committee approved the protocol. The fibrosis stage of the patients was assessed using liver biopsy and according to the METAVIR scoring system (5). IgG and albumin were depleted from these samples using the ProteoPrep Immunoaffinity Albumin and IgG Depletion kit (Sigma-Aldrich) according to the manufacturer's instructions. Briefly 20 μl of serum was diluted 5× in the equilibration buffer (low ionic strength Tris buffer, pH 7.4) and applied to the spin column followed by incubation for 5–10 min. After centrifugation (1 min at 3000 rpm), the eluate was reapplied to maximize binding. The unbound proteins were flushed off the column with 125 μl of the equilibration buffer. The serum depleted of IgG and albumin (225 μl) was stored at −20 °C. Subsequently the column-bound proteins were eluted with 2 × 150 μl of 40 mm Tris buffer, pH 10.4, containing 7 m urea, 2 m thiourea, and 1% C7BzO (3-(4-heptyl)phenyl-3-hydroxypropyldimethylammoniopropanesulfonate) detergent. Eluate was stored at 4 °C. Before measuring protein concentration, serum, IgG/albumin-depleted serum, and IgG/albumin fractions were diluted 100×, 4×, and 5×, respectively, with the commercial N-diluent buffer (Dade Behring) to neutralize fractions.
N-Glycan Analysis of the Three Fractions Obtained after IgG/Albumin Depletion of Sera—
Our optimized 96-well on-membrane deglycosylation method (23) was used to prepare APTS-labeled N-glycans from the three fractions obtained from each serum sample. Samples were then digested with A. ureafaciens sialidase (Glyko): 0.5 μl of sample was added to 2 μl of 5 mm NH4Ac, pH 5, containing 2 milliunits of sialidase and incubated overnight at 37 °C. Mixtures were evaporated to dryness under vacuum and reconstituted in 10 μl of Milli-Q water. The samples were injected in the capillaries of an ABI 3130 DNA sequencer (Applied Biosystems,) for 80 s at 1200 V and further analyzed as described previously (23).
Statistical Analysis of the Data—
To assess the classification efficiency of the different biomarkers, receiver operating characteristic (ROC) curve analysis was done to determine the area under curve (AUC) values. All error bars, unless otherwise stated, represent 95% confidence intervals (CI) for the mean. To determine whether components were correlated with each other, we used the two-tailed Spearman rank correlation test. All analyses were performed with the SPSS 15.0 software (SPSS Inc., Chicago, IL).
RESULTS
Performance of GlycoFibroTest as Liver Fibrosis Correlate—
We developed a protocol that is optimized to prepare and analyze the N-glycans derived from total serum protein (N-glycome) as described under “Experimental Procedures” (Fig. 1). Briefly an SDS-containing buffer is added to the serum to denature the proteins, and subsequently the N-glycans are specifically released from their carrier proteins by adding peptide N-glycosidase F. Finally the N-glycans are desialylated (to simplify the final N-glycan profile) and labeled with an APTS fluorophore, allowing separation and detection on currently available DNA sequencers. During liver fibrosis progression, the amount of NGA2FB increases and the NA3 concentration decreases in the N-glycome (see Fig. 2 for nomenclature). We renamed the log-transformed ratio between NGA2FB and NA3 as GlycoFibroTest. Mean GlycoFibroTest and hyaluronate values appear to increase in an exponential fashion with increasing fibrosis stage (Fig. 3) with substantial increases from F1 onward. This is an expected feature for a marker that can monitor fibrosis progression. Indeed the amount of fibrotic tissue accumulates progressively more rapidly during fibrosis progression. GlycoFibroTest and hyaluronate correlate equally well with the METAVIR classification (F1–F4) of liver fibrosis (ρ = 0.44 and 0.45, respectively; correlations are significant at the 0.01 level).
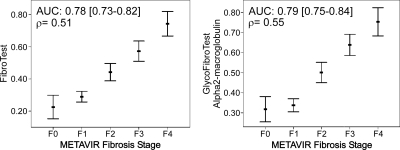
Fig. 3.
Correlation of GlycoFibroTest, log(hyaluronate), and α2-macroglobulin with METAVIR fibrosis stage. GlycoFibroTest is the log-transformed ratio between NGA2FB and NA3. The graphs represent 95% CI error bars of the mean. The AUC (95% CI) for diagnosing F2–F4 and the correlation with F1–F4 is given in the top corner (Spearman ρ).
The mean FibroTest values for the consecutive fibrosis stages appear to increase in a sigmoidal way with the transition between F1 and F2 (Fig. 4). This is due to the binary logistic regression model on which the seven-parameter test is based to distinguish between F0–F1 and F2–F4 (i.e. clinically significant fibrosis). To assess the contribution of each of the individual parameters to making this distinction, we determined the area under the ROC curves. The mean AUC (with 95% CI) for diagnosing significant fibrosis was 0.78 (0.73–0.82) for FibroTest. For α2-macroglobulin, one of the FibroTest components, the AUC was 0.75 (0.70–0.80) (Fig. 3; other FibroTest parameters are in the supplemental figure). Thus, the diagnostic power of α2-macroglobulin is very similar to that of the complete algorithm, and the averages of α2-macroglobulin values in the consecutive fibrosis stages appear to increase in a sigmoidal fashion (Fig. 3): α2-macroglobulin concentrations increase sharply from F1 to F2 but do not increase much in the later stages of fibrosis. By combining the other FibroTest components with α2-macroglobulin, the averages of the combined biomarker in the consecutive fibrosis stages map better to a line. FibroTest correlates moderately well with the METAVIR fibrosis stage in the F1–F4 area (ρ = 0.51) as equally good as GlycoFibroTest and hyaluronate.
Fig. 4.
FibroTest and the combination of GlycoFibroTest with α2-macroglobulin in a binary logistic regression model are plotted against METAVIR fibrosis stage. The model was constructed using clinically significant fibrosis (F2–F4) as the dependent variable. The error bars represent 95% confidence intervals for the mean. The AUC (95% CI) to distinguish significant fibrosis (F2–F4) from insignificant fibrosis (F0–F1) is given together with the Spearman ρ correlation (F1–F4).
We created multivariate logistic regression models with GlycoFibroTest, FibroTest components, and hyaluronic acid in an attempt to create better predictors for significant fibrosis. Nevertheless these models were not significantly better than the individual biomarkers (data not shown). However, we optimized GlycoFibroTest, a very useful marker to monitor fibrosis progression from F1 onward by combining it with α2-macroglobulin (Fig. 3), which is slightly better at distinguishing F0–F1 from F2–F4 (AUC = 0.75 versus 0.71) in a binary logistic regression model (Fig. 4). This model yielded a biomarker with an area under the ROC curve of 0.79 (0.75–0.84) for diagnosing significant fibrosis and with a F1–F4 correlation at least as good as that of FibroTest (ρ = 0.55 versus 0.51). A second study will be necessary to verify the additional value of this biomarker compared with GlycoFibroTest alone. Poynard et al. (29) showed that the prevalence of fibrosis stages has an effect on the AUC for diagnosing ≥F2 and suggested the DANA (difference advanced − non-advanced fibrosis stage analysis) index to model a homogeneous liver fibrosis stage distribution (i.e. prevalence of each stage: 20%). Table I lists the AUCs estimated according to DANA.
Table I.
AUCs for diagnosing clinically significant fibrosis (F2–F4) determined using the DANA index
DANA is a method to model a uniform fibrosis stage prevalence in a study cohort with a skewed fibrosis distribution and can be used to compare the AUCs of biomarkers from different studies.
| Biomarkers | AUC (≥F2) |
|---|---|
| GlycoFibroTest | 0.75 |
| α2-Macroglobulin | 0.78 |
| log(hyaluronate) | 0.78 |
| FibroTest | 0.83 |
| Binary logistic regression model (α2-macroglobulin + GlycoFibroTest) | 0.84 |
IgG: Carrier of Undergalactosylated Biantennary Glycans of GlycoFibroTest—
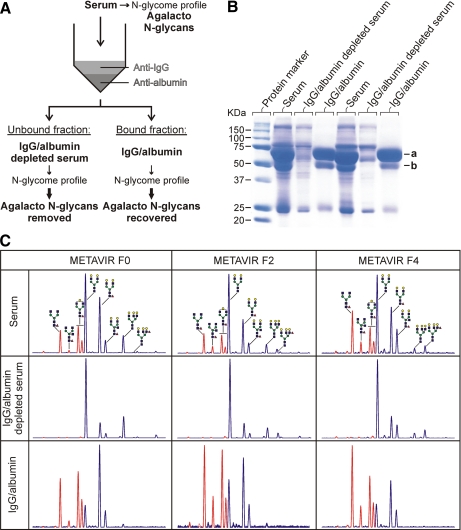
In the course of our studies, we noted that agalacto N-glycans in serum were much more difficult to regalactosylate enzymatically when still bound to their carrier proteins than when they had been released from the proteins by peptide N-glycosidase F treatment. Thus, the accessibility of these N-glycan chains seems to be hindered. As the N-glycans on the Fc part of IgG are often only partially galactosylated and as these N-glycans are indeed partially shielded by the IgG protein chain (30), we investigated the extent to which the agalacto N-glycans in the serum of fibrosis patients were linked to IgG. We approached this question by purifying out IgG and albumin, carefully quantifying the proteins in the different fractions obtained, and correlating the results with N-glycan profiles obtained from these fractions (Fig. 5). ProteoPrep Immunoaffinity Albumin and IgG Depletion kit columns contain two media with small antibody fragments (12 kDa) that specifically bind to albumin and IgG. Removal of albumin does not interfere with the aim of this study because it is not glycosylated. SDS-PAGE (Fig. 5B) shows that the albumin (66-kDa) and IgG heavy chain (50-kDa) bands are absent in the depleted samples, whereas no other proteins are detected in the purified fractions. To further assess purification efficiency, absolute protein quantification was done on sensitive and clinically validated analyzers. This showed that the purification was reproducible (σ = 4–6% for IgG, IgA, and transferrin), and only albumin recovery was more variable (σ = 12%). Transferrin and IgA were used as representatives of the non-IgG glycoprotein population and were recovered from the columns with the same efficiency (75 ± 5 and 81 ± 6%, respectively); thus we only looked at the effect of removing IgG and albumin. The purification data showed that, on average, 99% (σ = 1%) of IgG was removed from the sera, and this resulted in an almost total depletion of the undergalactosylated biantennary glycans in the N-glycome (Fig. 5C). Noteworthy is that all agalacto glycans in the cirrhosis sample shown in Fig. 5C were removed after IgG/albumin depletion, although IgA constitutes 29% of the proteins in this fraction (serum IgA concentration was 6.1 g/liter). For the IgG fractions, an average purity of 94% (σ = 3%) was obtained. In the corresponding sugar profiles, the pattern of undergalactosylated biantennary glycans corresponds to the glycans depleted from the serum. These findings show that IgG is the only major protein carrying these structures.
Fig. 5.
Strategy for identifying the carrier proteins of agalacto N-glycans of GlycoFibroTest (A), SDS-PAGE analysis of two serum samples before and after albumin/IgG removal (B), and N-glycan profile (DSA-FACE) of each fraction after desialylation (C). A, IgG and albumin were removed from sera by affinity chromatography. The N-glycans from each fraction were analyzed as described under “Experimental Procedures.” B, albumin (a) and IgG (b; heavy chain) were removed from sera as described under “Experimental Procedures.” The equivalent of 0.5 μl of serum of each fraction was separated by 10% SDS-PAGE and stained with Coomassie stain. C, sugars lacking one or more galactose residues are marked in red. Yellow circle, galactose; blue square, N-acetylglucosamine; green circle, mannose; red triangle, fucose.
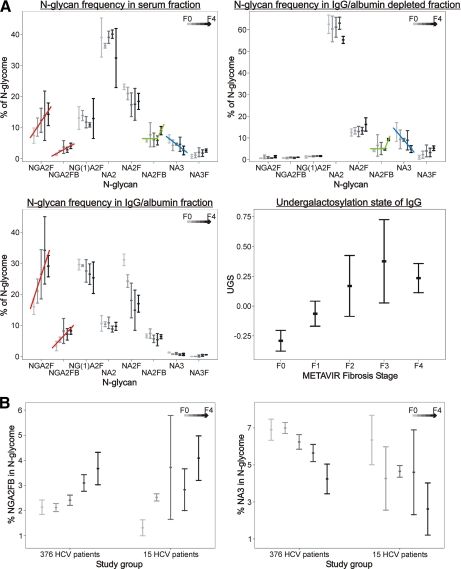
In the sera we see that NGA2F and NGA2FB gradually increase as liver fibrosis progresses (Fig. 6A, red lines). This is reproduced in the IgG fraction but not in the IgG/albumin-depleted serum. The mean undergalactosylation state of IgG (i.e. the log-transformed ratio between NGA2F and NA2F) increases in a linear fashion over the consecutive fibrosis stages and reaches a maximum in the F3–F4 area. Another observation is that NA2FB, which is specifically up-regulated in serum of cirrhosis patients (GlycoCirrhoTest (22)), is present in higher abundance only in IgG/albumin-depleted serum of cirrhosis patients, whereas this is not the case for the IgG fractions (Fig. 6A, green lines). As most of the non-IgG glycoproteins in serum are produced by hepatocytes, this finding is in line with the report of Ishibashi et al. (31) that the activity of N-acetylglucosaminyltransferase III, which is responsible for this bisecting N-acetylglucosamine (GlcNAc) modification on NA2FB, is increased in liver nodules of cirrhosis patients. Finally the decrease in fully galactosylated triantennary N-glycans (NA3) in serum during liver fibrosis progression can be seen in the IgG/albumin-depleted fraction but not on IgG (Fig. 6A, blue lines). Indeed triantennary structures on human IgG are rare (32).
Fig. 6.
N-Glycan distribution in each fraction and correlation of the undergalactosylation state of IgG with METAVIR fibrosis stage (A) GlycoFibroTest components plotted against METAVIR fibrosis stage: comparison between the study groups (B). A, the total signal of the 12 analyzed glycans was used to normalize the abundance of each glycan species. The undergalactosylation state of IgG is the log-transformed ratio between the core-fucosylated biantennary agalacto sugar (NGA2F) and its galactosylated counterpart (NA2F). B, the concentrations of both GlycoFibroTest components (NGA2FB and NA3) in the serum N-glycome were plotted against the METAVIR classification of liver fibrosis for two patient populations. The first population consists of 376 HCV patients (main study group); the second consists of 15 HCV patients (IgG study group). For the analyzed N-glycans, a similar trend is observed in both populations. The error bars represent the 2× S.E. Lightest gray, F0; light gray, F1; medium gray, F2; darkest gray, F3; black, F4.
DISCUSSION
Liver biopsy is often indicated for newly diagnosed chronic liver disease. When used in combination, non-invasive markers such as GlycoCirrhoTest, FibroTest, and hyaluronate are reliable tools for diagnosis of liver cirrhosis in a large portion of the patients, even at an early stage of cirrhosis (8, 22), and to exclude the presence of significant fibrosis in a fraction of the patients. However, we believe that the true value of non-invasive surrogate markers does not lie in avoiding that first liver biopsy but in monitoring the progression of the disease over the many years that often lie between first diagnosis and serious complications. Such monitoring would be valuable for making clinical decisions. For example, continuous progression of fibrosis in treated patients would indicate non-responders and would necessitate more intensive monitoring of those patients; early detection of clinically non-overt (compensated) cirrhosis will allow screening of this HCC risk group and hopefully detection of HCC when surgery is still beneficial. For monitoring liver fibrosis progression in chronic HCV patients, our data strongly suggest that GlycoFibroTest, FibroTest, and hyaluronate have almost identical value (very similar correlation to the F1–F4 METAVIR stages). Longitudinal studies are ongoing to monitor F1 and F2 patients for several years to confirm these notions. Moreover ongoing studies show that GlycoFibroTest can be applied to other underlying causes of liver fibrosis, such as non-alcoholic steatohepatitis (37) and hepatitis B virus infection.2
In this study, we demonstrated that the undergalactosylated biantennary glycans that allow the follow-up of liver fibrosis (GlycoFibroTest) are present exclusively on IgG, whereas the triantennary glycans, the abundance of which decreases in fibrosis, are present on non-IgG proteins, most of which are liver-produced. As IgG undergalactosylation also occurs in other chronic necroinflammatory diseases, such as rheumatoid arthritis (RA) (33), one might expect interference of these diseases with GlycoFibroTest. However, GlycoFibroTest is based on the ratio between a bisecting GlcNAc-modified agalacto glycan and a triantennary glycan. The latter decreases with fibrosis but not in diseases such as RA. Moreover the bisecting GlcNAc modification on IgG glycans is up-regulated in fibrosis but not in RA (34). Furthermore IgG concentrations tend to progressively increase with fibrosis stage (35), augmenting the fraction of N-glycans in the total serum N-glycome that are derived from IgG. No such alteration in IgG concentration occurs in RA. The consequence of these three factors is that the GlycoFibroTest values for 24 RA patients overlapped only with F0–F1 patients in our previous study (22), and thus interference of such a disease with the monitoring of fibrosis progression is small if any. Fibrosis biomarkers that rely only on IgG undergalactosylation (36) would likely not benefit from these specificity advantages of GlycoFibroTest in patients with another necroinflammatory disease. The undergalactosylation of IgG prompts for further research so that we fully understand the pathophysiology that underlies the glycosylation changes in chronic liver disease.
However, the glycomics-based biomarkers have some advantages over the other surrogate markers. The robust and sensitive method (DSA-FACE) (23) by which the glycans are analyzed allows standardized implementation of this technology in any clinical laboratory that has a standard DNA sequencer, and we are working toward this goal. The assay that we used to prepare and label N-glycans only requires a thermocycler and can be learned by a laboratory technician in short notice. Moreover still simpler assays are under development. As the N-glycome profile (renamed as GlycoHepatoTest) provides useful information over the whole spectrum of chronic liver disease (GlycoFibroTest, GlycoCirrhoTest, and GlycoHCCTest), we envisage its use in the following way: (i) monitoring of liver fibrosis progression using GlycoFibroTest alone or in combination with α2-macroglobulin; (ii) improved diagnosis of early stage (compensated) liver cirrhosis using GlycoCirrhoTest in combination with FibroTest (22), hyaluronate, or APRI; and (iii) improved diagnosis of HCC in cirrhotic patients using GlycoHCCTest in combination with α-fetoprotein (24). In this way, GlycoHepatoTest strongly complements the current arsenal of well validated single parameter clinical chemistry used in the hepatology field without having to resort to complicated multivariate statistical models.
Supplementary Material
Footnotes
Published, MCP Papers in Press, January 29, 2009, DOI 10.1074/mcp.M800470-MCP200
The abbreviations used are: HCC, hepatocellular carcinoma; APRI, aspartate transaminase to platelets ratio index; APTS, 8-aminopyrene-1,3,6-trisulfonic acid; AUC, area under curve; CI, confidence interval; DANA, difference advanced − non-advanced fibrosis stage analysis; DSA-FACE, DNA sequencer-assisted fluorophore-assisted carbohydrate electrophoresis; GlcNAc, N-acetylglucosamine; HCV, hepatitis C virus; RA, rheumatoid arthritis; ROC, receiver operating characteristic; IUBMB, International Union of Biochemistry and Molecular Biology.
Q. Xie, C. F. Gao, X. E. Liu, S. Dewaele, H. L. Gui, H. Wang, L. Wang, H. Zhuang, C. Libert, R. Contreras, and C. Chen, unpublished data.
This work was supported by a Concerted Research Program grant from Ghent University, by Marie Curie Excellence Grant 014292 (European Union Framework Programme 6), and by a postdoctoral grant from Institute for the Promotion of Innovation by Science and Technology in Flanders (to W. L.).
The on-line version of this article (available at http://www.mcponline.org) contains supplemental material.
REFERENCES
- 1.Bravo, A. A., Sheth, S. G., and Chopra, S. ( 2001) Liver biopsy. N. Engl. J. Med. 344, 495–500 [DOI] [PubMed] [Google Scholar]
- 2.Poynard, T., Munteanu, M., Imbert-Bismut, F., Charlotte, F., Thabut, D., Le Calvez, S., Messous, D., Thibault, V., Benhamou, Y., Moussalli, J., and Ratziu, V. ( 2004) Prospective analysis of discordant results between biochemical markers and biopsy in patients with chronic hepatitis C. Clin. Chem. 50, 1344–1355 [DOI] [PubMed] [Google Scholar]
- 3.Friedman, S. L. ( 2003) Liver fibrosis—from bench to bedside. J. Hepatol. 38, Suppl. 1, S38–S53 [DOI] [PubMed] [Google Scholar]
- 4.Sato, Y., Murase, K., Kato, J., Kobune, M., Sato, T., Kawano, Y., Takimoto, R., Takada, K., Miyanishi, K., Matsunaga, T., Takayama, T., and Niitsu, Y. ( 2008) Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat. Biotechnol. 26, 431–442 [DOI] [PubMed] [Google Scholar]
- 5.Bedossa, P., Bioulac-Sage, P., Callard, P., Chevallier, M., Degott, C., Deugnier, Y., Fabre, M., Reynés, M., Voigt, J. J., and Zafrani, E. S. ( 1994) Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology 20, 15–20 [PubMed] [Google Scholar]
- 6.Kuper, H., Ye, W., Broome, U., Romelsjo, A., Mucci, L. A., Ekbom, A., Adami, H. O., Trichopoulos, D., and Nyren, O. ( 2001) The risk of liver and bile duct cancer in patients with chronic viral hepatitis, alcoholism, or cirrhosis. Hepatology 34, 714–718 [DOI] [PubMed] [Google Scholar]
- 7.Laurent, T. C., and Fraser, J. R. ( 1992) Hyaluronan. FASEB J. 6, 2397–2404 [PubMed] [Google Scholar]
- 8.Guechot, J., Laudat, A., Loria, A., Serfaty, L., Poupon, R., and Giboudeau, J. ( 1996) Diagnostic accuracy of hyaluronan and type III procollagen amino-terminal peptide serum assays as markers of liver fibrosis in chronic viral hepatitis C evaluated by ROC curve analysis. Clin. Chem. 42, 558–563 [PubMed] [Google Scholar]
- 9.George, J., Tsutsumi, M., and Takase, S. ( 2004) Expression of hyaluronic acid in N-nitrosodimethylamine induced hepatic fibrosis in rats. Int. J. Biochem. Cell Biol. 36, 307–319 [DOI] [PubMed] [Google Scholar]
- 10.Naveau, S., Poynard, T., Benattar, C., Bedossa, P., and Chaput, J. C. ( 1994) α-2-Macroglobulin and hepatic fibrosis. Diagnostic interest. Dig. Dis. Sci. 39, 2426–2432 [DOI] [PubMed] [Google Scholar]
- 11.Wai, C. T., Greenson, J. K., Fontana, R. J., Kalbfleisch, J. D., Marrero, J. A., Conjeevaram, H. S., and Lok, A. S. ( 2003) A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 38, 518–526 [DOI] [PubMed] [Google Scholar]
- 12.Aster, R. H. ( 1966) Pooling of platelets in the spleen: role in the pathogenesis of “hypersplenic” thrombocytopenia. J. Clin. Investig. 45, 645–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawasaki, T., Takeshita, A., Souda, K., Kobayashi, Y., Kikuyama, M., Suzuki, F., Kageyama, F., Sasada, Y., Shimizu, E., Murohisa, G., Koide, S., Yoshimi, T., Nakamura, H., and Ohno, R. ( 1999) Serum thrombopoietin levels in patients with chronic hepatitis and liver cirrhosis. Am. J. Gastroenterol. 94, 1918–1922 [DOI] [PubMed] [Google Scholar]
- 14.Kamimoto, Y., Horiuchi, S., Tanase, S., and Morino, Y. ( 1985) Plasma clearance of intravenously injected aspartate aminotransferase isozymes: evidence for preferential uptake by sinusoidal liver cells. Hepatology 5, 367–375 [DOI] [PubMed] [Google Scholar]
- 15.Poordad, F. F. ( 2004) FIBROSpect II: a potential noninvasive test to assess hepatic fibrosis. Expert Rev. Mol. Diagn. 4, 593–597 [DOI] [PubMed] [Google Scholar]
- 16.Christensen, C., Bruden, D., Livingston, S., Deubner, H., Homan, C., Smith, K., Oh, E., Gretch, D., Williams, J., and McMahon, B. ( 2006) Diagnostic accuracy of a fibrosis serum panel (FIBROSpect II) compared with Knodell and Ishak liver biopsy scores in chronic hepatitis C patients. J. Viral Hepat. 13, 652–658 [DOI] [PubMed] [Google Scholar]
- 17.Zaman, A., Rosen, H. R., Ingram, K., Corless, C. L., Oh, E., and Smith, K. ( 2007) Assessment of FIBROSpect II to detect hepatic fibrosis in chronic hepatitis C patients. Am. J. Med. 120, 280.e9–280.e14 [DOI] [PubMed] [Google Scholar]
- 18.Imbert-Bismut, F., Ratziu, V., Pieroni, L., Charlotte, F., Benhamou, Y., and Poynard, T. ( 2001) Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet 357, 1069–1075 [DOI] [PubMed] [Google Scholar]
- 19.Rosenthal-Allieri, M. A., Peritore, M. L., Tran, A., Halfon, P., Benzaken, S., and Bernard, A. ( 2005) Analytical variability of the Fibrotest proteins. Clin. Biochem. 38, 473–478 [DOI] [PubMed] [Google Scholar]
- 20.Sandrin, L., Fourquet, B., Hasquenoph, J. M., Yon, S., Fournier, C., Mal, F., Christidis, C., Ziol, M., Poulet, B., Kazemi, F., Beaugrand, M., and Palau, R. ( 2003) Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med. Biol. 29, 1705–1713 [DOI] [PubMed] [Google Scholar]
- 21.Callewaert, N., Geysens, S., Molemans, F., and Contreras, R. ( 2001) Ultrasensitive profiling and sequencing of N-linked oligosaccharides using standard DNA-sequencing equipment. Glycobiology 11, 275–281 [DOI] [PubMed] [Google Scholar]
- 22.Callewaert, N., Van Vlierberghe, H., Van Hecke, A., Laroy, W., Delanghe, J., and Contreras, R. ( 2004) Noninvasive diagnosis of liver cirrhosis using DNA sequencer-based total serum protein glycomics. Nat. Med. 10, 429–434 [DOI] [PubMed] [Google Scholar]
- 23.Laroy, W., Contreras, R., and Callewaert, N. ( 2006) Glycome mapping on DNA sequencing equipment. Nat. Protoc. 1, 397–405 [DOI] [PubMed] [Google Scholar]
- 24.Liu, X. E., Desmyter, L., Gao, C. F., Laroy, W., Dewaele, S., Vanhooren, V., Wang, L., Zhuang, H., Callewaert, N., Libert, C., Contreras, R., and Chen, C. ( 2007) N-Glycomic changes in hepatocellular carcinoma patients with liver cirrhosis induced by hepatitis B virus. Hepatology 46, 1426–1435 [DOI] [PubMed] [Google Scholar]
- 25.Halfon, P., Bourliere, M., Deydier, R., Botta-Fridlund, D., Renou, C., Tran, A., Portal, I., Allemand, I., Bertrand, J. J., Rosenthal-Allieri, A., Rotily, M., Sattonet, C., Benderitter, T., Saint Paul, M. C., Bonnot, H. P., Penaranda, G., Degott, C., Masseyeff, M. F., and Ouzan, D. ( 2006) Independent prospective multicenter validation of biochemical markers (fibrotest-actitest) for the prediction of liver fibrosis and activity in patients with chronic hepatitis C: the fibropaca study. Am. J. Gastroenterol. 101, 547–555 [DOI] [PubMed] [Google Scholar]
- 26.Regev, A., Berho, M., Jeffers, L. J., Milikowski, C., Molina, E. G., Pyrsopoulos, N. T., Feng, Z. Z., Reddy, K. R., and Schiff, E. R. ( 2002) Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am. J. Gastroenterol. 97, 2614–2618 [DOI] [PubMed] [Google Scholar]
- 27.Fink, P. C., Romer, M., Haeckel, R., Fateh-Moghadam, A., Delanghe, J., Gressner, A. M., and Dubs, R. W. ( 1989) Measurement of proteins with the Behring Nephelometer. A multicentre evaluation. J. Clin. Chem. Clin. Biochem. 27, 261–276 [PubMed] [Google Scholar]
- 28.Orsonneau, J. L., Douet, P., Massoubre, C., Lustenberger, P., and Bernard, S. ( 1989) An improved pyrogallol red-molybdate method for determining total urinary protein. Clin. Chem. 35, 2233–2236 [PubMed] [Google Scholar]
- 29.Poynard, T., Halfon, P., Castera, L., Munteanu, M., Imbert-Bismut, F., Ratziu, V., Benhamou, Y., Bourliere, M., and de Ledinghen, V. ( 2007) Standardization of ROC curve areas for diagnostic evaluation of liver fibrosis markers based on prevalences of fibrosis stages. Clin. Chem. 53, 1615–1622 [DOI] [PubMed] [Google Scholar]
- 30.Krapp, S., Mimura, Y., Jefferis, R., Huber, R., and Sondermann, P. ( 2003) Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. J. Mol. Biol. 325, 979–989 [DOI] [PubMed] [Google Scholar]
- 31.Ishibashi, K., Nishikawa, A., Hayashi, N., Kasahara, A., Sato, N., Fujii, S., Kamada, T., and Taniguchi, N. ( 1989) N-Acetylglucosaminyltransferase III in human serum, and liver and hepatoma tissues: increased activity in liver cirrhosis and hepatoma patients. Clin. Chim. Acta 185, 325–332 [DOI] [PubMed] [Google Scholar]
- 32.Raju, T. S., Briggs, J. B., Borge, S. M., and Jones, A. J. ( 2000) Species-specific variation in glycosylation of IgG: evidence for the species-specific sialylation and branch-specific galactosylation and importance for engineering recombinant glycoprotein therapeutics. Glycobiology 10, 477–486 [DOI] [PubMed] [Google Scholar]
- 33.Parekh, R. B., Dwek, R. A., Sutton, B. J., Fernandes, D. L., Leung, A., Stanworth, D., Rademacher, T. W., Mizuochi, T., Taniguchi, T., Matsuta, K., Takeuchi, F., Nagoino, Y., Miyamoto, T., and Kobaka, A. ( 1985) Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature 316, 452–457 [DOI] [PubMed] [Google Scholar]
- 34.Van Beneden, K., Coppieters, K., Laroy, W., De Keyser, F., Hoffman, I. E., Van den Bosch, F., Vander Cruyssen, B., Drennan, M., Jacques, P., Rottiers, P., Verbruggen, G., Contreras, R., Callewaert, N., and Elewaut, D. ( 2009) Reversible changes in serum immunoglobulin galactosylation during the immune response and treatment of inflammatory auto-immune arthritis. Ann. Rheum. Dis., in press [DOI] [PubMed]
- 35.Watt, K., Uhanova, J., Gong, Y., Kaita, K., Doucette, K., Pettigrew, N., and Minuk, G. Y. ( 2004) Serum immunoglobulins predict the extent of hepatic fibrosis in patients with chronic hepatitis C virus infection. J. Viral Hepat. 11, 251–256 [DOI] [PubMed] [Google Scholar]
- 36.Mehta, A. S., Long, R. E., Comunale, M. A., Wang, M., Rodemich, L., Krakover, J., Philip, R., Marrero, J. A., Dwek, R. A., and Block, T. M. ( 2008) Increased levels of galactose-deficient anti-Gal immunoglobulin G in the sera of hepatitis C virus-infected individuals with fibrosis and cirrhosis. J. Virol. 82, 1259–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen, C., Schmilovitz-Weiss, H., Liu, X. E., Pappo, O., Halpern, M., Sulkes, J., Braun, M., Cohen, M., Barak, N., Tur-Kaspa, R., Vanhooren, V., Van Vlierberghe, H., Libert, C., Contreras, R., and Ben-Ari, Z. ( 2009) Serum protein N-glycans profiling for the discovery of potential biomarkers for nonalcoholic steatohepatitis. J. Proteome Res. 8, 463–470 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.