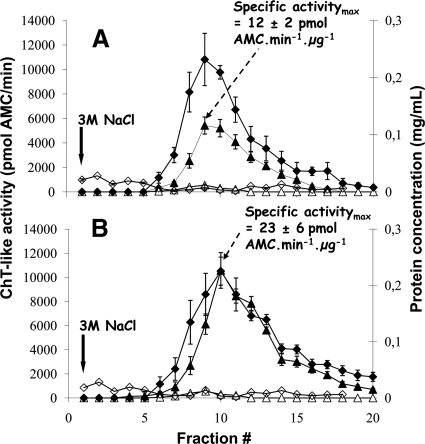
Fig. 2.
Proteasome purification from human erythrocytes without (A) or with (B) in vivo formaldehyde cross-linking. Proteasome complexes were purified from human erythrocytes by affinity chromatography with the mouse IgG1 monoclonal antibody MCP21 coupled to Sepharose beads. A control purification was performed using the mouse IgG1 monoclonal antibody OX8 directed against rat CD8α. Proteins were cross-linked in vivo by incubation of human erythrocytes with 1% formaldehyde as indicated under “Experimental Procedures.” After incubation of 50 ml of erythrocyte lysate with either the MCP21-Sepharose or the OX8-Sepharose, proteins interacting with the beads were eluted by a saline step of 3 m NaCl. The eluted fractions were analyzed for their protein concentrations as well as for their proteasome contents. Statistical results were obtained from three independent experiments for each condition (formaldehyde-treated or not). Error bars indicate standard deviations (n = 3). ♦ and ⋄ represent protein concentrations in the fractions from the MCP21-Sepharose beads and from the OX8-Sepharose beads, respectively. ▴ and ▵ represent the in vitro ChT-like activity without and with 10 μm lactacystin, respectively, in the fractions eluted from the MCP21-Sepharose beads. No ChT-like activity could be detected in the fractions from the OX8 negative control experiment.