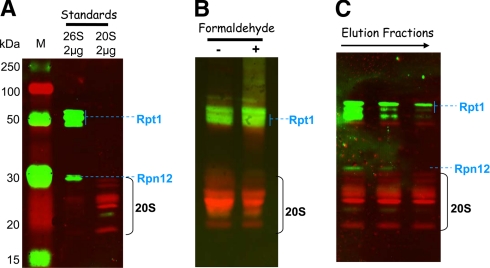
Fig. 3.
Detection of 20 S core particle and 19 S activators in the purified proteasome preparations. Proteins separated by SDS-PAGE were transferred to a nitrocellulose membrane: 2 μg of commercial 20 S and 26 S proteasome from human erythrocytes as standards (A), 20 μg of total proteins from fractions containing the maximal ChT-like activity (fractions 9 and 10 from purifications without and with formaldehyde cross-linking, respectively) (B), and 10 μg of estimated 20 S proteasome (based on the ChT-like activity measurement) from fractions 8, 9, and 10 from the purification without formaldehyde cross-linking (C). Mouse monoclonal primary antibodies against 19 S proteasome subunits Rpt1 and Rpn12 and rabbit polyclonal antibodies against 20 S core subunits were used for the immunoblot staining. ECL Plex CyDye-conjugated antibodies, goat α-mouse IgG-Cy3 and goat α-rabbit IgG-Cy5, were used as secondary antibodies. The detection was performed using the Typhoon Trio fluorescence scanner at 532 nm excitation and 580 nm emission for the Cy3-conjugated antibody and 633 nm excitation and 670 nm emission for the Cy5-conjugated antibody. Lane M, molecular mass markers.