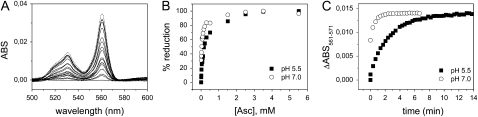
Figure 7.
Reaction of ascorbate with purified recAIR12. A, Reduced minus oxidized optical spectra were recorded during titration of recAIR12 (1.6 μm) in 50 mm MES-KOH, pH 5.5, with ascorbate at the following final concentrations: 5, 10, 15, 25, 40, 55, 70, 90, 110, 160, 210, 310, 510, 1,510, 2,510, 3,510, and 5,510 μm. Finally, dithionite (a few grains) was added (dashed line). B, The percentage increase in absorbance at 561 nm is plotted as a function of ascorbate concentration for recAIR12 at pH 5.5 (same as in A; black squares) and recAIR12 (4.2 μm) equilibrated with 50 mm Tris-HCl, pH 7.0, 150 mm KCl (white circles, titrated with ascorbate at the same concentrations used for the sample at pH 5.5). C, Time courses of the absorbance change at 561 nm (relative to isosbestic point at 571 nm) upon reduction with ascorbate of purified recAIR12, at pH 5.5 (black squares) and pH 7.0 (white circles). Ascorbate (0.3 mm final concentration) was added to recAIR12 (0.7 μm) and absorption spectra were recorded approximately every 20 s. ABS, Absorbance.