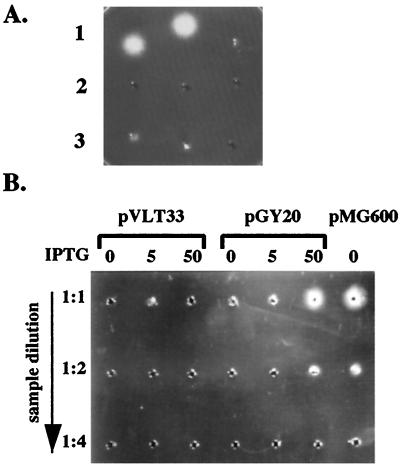
Figure 3.
YplA is an extracellular protein whose production requires expression of the flagellar regulon. (A) Concentrated whole-cell lysates and culture supernatants of strain JB580v grown in LB or T medium were examined for phospholipase activity by a radial diffusion assay. A positive reaction resulted in the formation of a precipitate emanating from the sample well in gels containing the artificial substrate Tween 80. Each well contained an equivalent of cells or supernatants from 1.5 ml culture at an OD600 of 3.0. Row 1, culture supernatant after growth in T medium (Left), culture supernatant after growth in T medium and preheated to 90°C for 10 min (Center), and cell lysate after growth in T medium (Right). Row 2, culture supernatant after growth in LB (Left), culture supernatant after growth in LB and preheated to 90°C for 10 min (Center), and cell lysate after growth in T medium and preheated to 90°C for 10 min (Right). Row 3, cell lysates after growth in LB (Left), cell lysate after growth in LB and preheated to 90°C for 10 min (Center), and buffer control (Right). (B) Phospholipase activity of secreted YplA was measured for strain GY460 containing plasmid pVLT33 (vector control), pGY20 (ptac-flhDC from Y. enterocolitica), and pMG600 (ptac-flhDC from S. liquefaciens). Conditions of the assay were as described in A. Each well contained concentrated culture supernatant for each strain grown in T medium, with the inducer IPTG at a concentration of 0, 5, or 50 μM (indicated at the top). From top to bottom each well was loaded with an increased dilution of each sample.