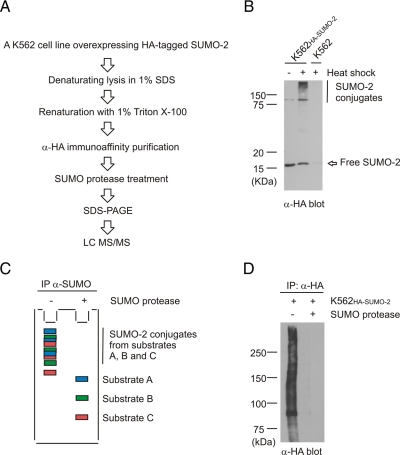
Fig. 1.
Strategy to identify heat shock-inducible SUMO-2 substrates. A, our purification is based on rapid inactivation of endogenous SUMO proteases and desumoylation of polysumoylated substrates. Endogenous SUMO proteases are inactivated immediately after cell lysis by boiling in 1% SDS. Renaturation is needed for antibody-based purification. Substrates are desumoylated on beads with recombinant Ulp-1 SUMO protease, separated by SDS-PAGE, and identified with mass spectrometry. B, K562HA-SUMO-2 cells express His-HA-tagged SUMO-2, which is conjugated to substrate proteins upon heat shock. Lysates from untreated or heat-shocked K562HA-SUMO-2 cells and heat-shocked parental K562 cells were separated by SDS-PAGE and detected with α-HA antibody. C, the presence of multiple sumoylation sites and the formation of SUMO-2 chains of various lengths result in substrates displaying a number of different sumoylation states. Different substrates are indicated by color codes. The different sumoylation states are split into discrete bands upon SDS-PAGE, and consequently a single band contains only a fraction of a multisumoylated substrate. Desumoylation prior to SDS-PAGE results in concentrated and well separated substrates. D, treatment with Ulp-1 SUMO protease on beads efficiently desumoylates substrates prior to SDS-PAGE. K562HA-SUMO-2 cells were heat-shocked, SUMO-2 conjugates were purified using α-HA-agarose, and the sample was split in two. Beads were treated with or without Ulp-1, washed with PBS, and boiled in Laemmli sample buffer. Proteins were separated by SDS-PAGE and blotted with α-HA antibody. IP, immunoprecipitate.