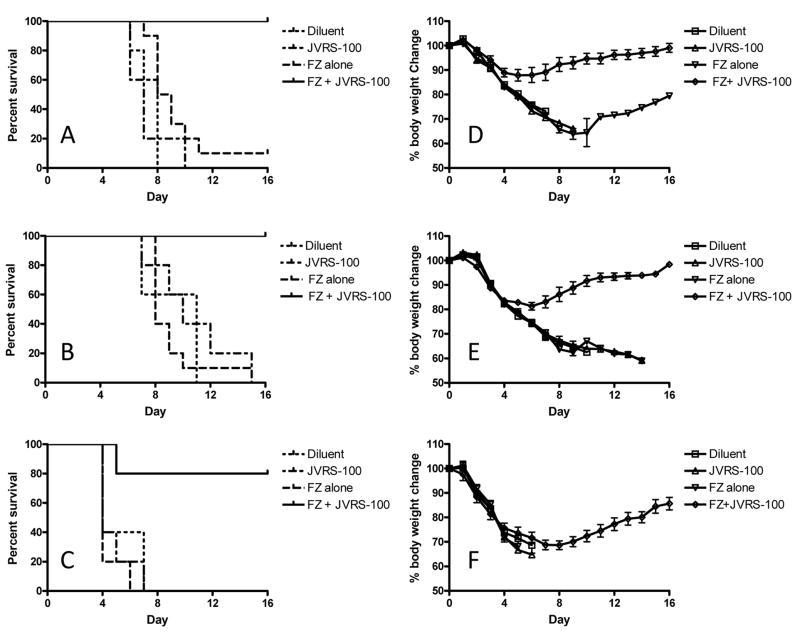
Fig. 4.
Fluzone® plus JVRS-100 immunization provides considerable cross-protection directed against influenza viral heterosubtypes. BALB/cJ mice (n=10 per group) were vaccinated with Fluzone® on Day 0 and 14 and challenged intranasally on day 28 with 2xLD50 of H1N1 (PR/8/34), H3N2 (HKx31), or influenza B (B/Lee/40) viruses. The groups were monitored for survival (panels A-C) and weight loss (panels D-F). Mice vaccinated with JVRS-100-Fluzone® compared to those receiving Fluzone® alone showed a significant enhancement in survival following viral challenge with (A) influenza A H1N1 (PR/8/34) ***P<0.0005; (B) influenza A H3N2 (HKx31) ***P<0.0005; or (C) influenza B (B/Lee/40) *P<0.05. P values were calculated using a log-rank (Mantel-Cox) test. Similarly, mice vaccinated with JVRS-100-Fluzone® compared to those receiving Fluzone® alone showed less weight loss following viral challenge with (D) influenza A H1N1 (PR/8/34) and (E) influenza A H3N2 (HKx31). There was not an improvement in weight loss following challenge with influenza B (B/Lee/40) (F), although the surviving mice (80%) recovered.