Abstract
The fluorogenic reagent Chromeo P465 is considered for analysis of proteins by capillary electrophoresis with laser-induced fluorescence detection. The reagent was first used to label α-lactalbumin; the product was analyzed by capillary zone electrophoresis in a sub-micellar sodium dodecyl sulfate (SDS) buffer. The product generated a set of equally spaced but poorly resolved peaks that formed a broad envelope with a net mobility of 4 × 10−4 cm2 V−1 s−1. The components of the envelope were presumably protein that had reacted with different numbers of labels. The mobility of these components decreased by roughly 1 % with the addition of each label. The signal increased linearly from 1.0 nM to 100 nM α-lactalbumin (r2 = 0.99), with a 3σ detection limit of 70 pM. We then considered the separation of a mixture of ovalbumin, α-chymotrypsinogen A, and αlactalbumin labeled with Chromeo P465; unfortunately, baseline resolution was not achieved with a borax/SDS buffer. Better resolution was achieved with N-cyclohexyl-2-aminoethanesulfonic acid/Tris/SDS/dextran capillary sieving electrophoresis; however, dye interactions with this buffer system produced a less than ideal blank.
Keywords: Chromeo P465, fluorogenic reagents, protein labeling, capillary electrophoresis, laser-induced fluorescence
1. Introduction
Capillary electrophoresis with laser-induced fluorescence detection is capable of rapid analysis of minute amounts of complex biological sample. Fluorogenic reagents, which undergo a large increase in fluorescence signal upon reacting with a primary amine, are particularly useful for the analysis of very low concentration protein samples. The preceding paper in this issue considered the reaction kinetics of three fluorogenic reagents, 3-(2-furoyl)quinoline-2-carboxaldehyde (FQ), Chromeo P465, and Chromeo P503 with α-lactalbumin [1]. These reagents were introduced by Novotny and Wolfbeis [2,3]. We observed that the reaction of FQ was sluggish compared with the Chromeo reagents but that the FQ reaction ultimately incorporated more labels at room temperature. We also observed that treatment of the protein with SDS at elevated temperature before the reaction resulted in the incorporation of a large number of Chromeo labels.
The data in the previous paper were generated by mass spectrometry. The mass spectrometry results inevitably showed the incorporation of a distribution of labels. For a protein with m possible labeling sites, there are 2m-1 possible fluorescent products [4]. These products can have dramatically different electrophoretic behavior, leading to an envelope of peaks producing complex electropherograms [4–6]. Most of our work has been with FQ, which converts cationic lysine residues into neutral products, which can be detected with exquisite sensitivity [7–10]. More importantly, we have shown that the incorporation of an anionic surfactant, such as SDS, results in the collapse of the multiple labeling envelope into a single, sharp peak [7].
This manuscript considers the electrophoretic and some spectroscopic properties of proteins labeled with Chromeo P465. The manufacturer’s literature quotes excitation and emission maxima of 644 nm and 732 nm, respectively, for the unconjugated dye and 465 nm and 629 nm, respectively, for the reaction product of the reagent with a protein. This reagent appears to be identical to the Py-5 reagent introduced by Wolfbeis [11]. In particular, we consider the detection limits and dynamic range, and electrophoretic behavior in different separation buffers. The following manuscript in this issue considers the behavior of Chromeo P503 [12].
2. Experimental
2.1 Chemicals
Chromeo P465 was purchased from Active Motif Chromeon (Tegernheim, Germany). Methanol 100.0 % purity was purchased from J.T. Baker (Phillipsburg, NJ, USA). Ovalbumin 98 % purity and α-lactalbumin ≥85 % purity by polyacrylamide gel electrophoresis were purchased from US Biological (Swampscott, MA, USA). Sodium hydrogencarbonate 99.5 % purity, sodium dodecyl sulfate (SDS) 99 % purity, N-cyclohexyl-2-aminoethanesulfonic acid (CHES) 99 % purity, sodium tetraborate decahydrate (borax) 99.5–100.5 % purity, dextran (from Leuconostoc mesenteroides, average molecular weight 400–500 kDa) and α-chymotrypsinogen A were purchased from Sigma (St. Louis, MO, USA). Tris(hydroxymethyl)aminomethane (Tris) was purchased from Invitrogen (Carlsbad, CA, USA). Sodium hydroxide 97 % was purchased from EMD Chemicals(Gibbstown, NJ, USA). UltraTrol Dynamic Pre-coat LN was purchased from Target Discovery (Palo Alto, CA, USA).
2.2 Solutions
All aqueous solutions were prepared with deionized water from a NANO Pure Diamond system (Barnstead International, Dubuque, IA, USA) and filtered before use through a 0.22 μm filter (Millipore, Bedford, MA, USA). 2.5 mM borax/3.5 mM SDS buffer (unadjusted pH 9.3) was prepared from 25 mM and 100 mM stocks, respectively. A buffer made from 100 mM CHES/100 mM Tris/3.5 mM SDS (unadjusted pH 8.9) was prepared from 0.50 M stocks of CHES and Tris and 100 mM SDS stock. Sieving buffer was prepared by adding sufficient solid dextran to CHES/Tris/SDS buffer to achieve a dextran concentration of 5 % w/v. Sodium hydrogencarbonate buffer was prepared at 0.10 M and adjusted to pH 9.0 with 1 M NaOH.
100 μM stock solutions of individual proteins were prepared in H2O and stored at −80°C as 20 μL aliquots. Prior to labeling, the protein stocks were diluted to the desired concentration in the reaction buffer, either hydrogencarbonate or borax/SDS.
P465 was prepared as a 1 mg/mL stock by dissolving the 5 mg as received in 5 mL methanol. 100 nmol aliquots of Chromeo P465 were prepared in 600 μL microcentrifuge tubes (Island Scientific, Bainbridge Island, WA, USA) by evaporating the methanol from 40 μL aliquots under vacuum using a Speed Vac (Savant Instruments, Farmingdale, NY, USA). Lyophilized Chromeo P465 aliquots were stored at −20 °C and reconstituted in methanol prior to use.
2.3 Labeling
Each day a fresh 100 nmol aliquot of Chromeo P465 was dissolved in 100 μL methanol. Care was taken to minimize exposure of the dye to light before and during reaction; dye solutions were kept on ice in darkness and reactions were carried out either in amber microcentrifuge tubes or in a dark chamber. The labeling reaction was initiated by adding sample to a solution of Chromeo P465 dissolved in either 0.10 M hydrogencarbonate (pH 9.0) or 2.5 mM borax/3.5 mM SDS (pH 9.3). The dye:protein ratio was maintained at no less than 10:1 in order to maintain a dye:lysine ratio of greater than 1:1.
2.4 Instrumentation
Capillary electrophoresis was performed on a locally constructed instrument employing post-column laser-induced fluorescence detection with a sheath-flow cuvette [10, 13–14]. Excitation was provided by a 473 nM laser (Lasermate Group, Pomona, CA, USA). Emission fluorescence was selected with a 630df30 bandpass filter (Omega Optical, Brattleboro, VA, USA). Photons were counted with an avalanche photodiode (EG&G Canada, Vaudreuil, Canada). Signal was recorded at 10 Hz.
Buffer pH was measured with an Accumet 13-620-223 Tris-compatible double junction electrode (Fisher Scientific, Pittsburgh, PA, USA).
2.5 Electrophoresis
Capillary zone electrophoresis (CZE) was carried out on a 25.7 cm long × 31 μm inner diameter uncoated fused silica capillary (Polymicro Technologies, Phoenix, AZ, USA) with a 2.5 mM borax/3.5 mM SDS run buffer. Sample was injected electrokinetically by applying 5 kV for 1 s. Separation was performed by applying 15 kV at positive polarity such that the cathode was at the detector.
Capillary sieving electrophoresis (CSE) was carried out on a 27.1 cm long × 31 μm i.d. fused silica capillary treated with UltraTrol LN to suppress the electroosmotic flow. The run buffer was 100 mM CHES/100 mM Tris/3.5 mM SDS/5 % w/v dextran (400 kDa-500 kDa MW). Sample was electrokinetically injected by applying 5 kV for 1 s. Separation was performed by applying 15 kV. Potentials were applied in negative polarity such that the anode was at the detector.
3. Results and discussion
3.1 Labeling conditions
Chromeo P465 was bright blue in solution. The dye turned yellow immediately when added to 0.1 M hydrogencarbonate and within 10 minutes when added to 2.5 mM borax/3.5 mM SDS buffer. Labeled protein turned the solution red. Chromeo P465 remained reactive for several hours when dissolved in these solutions, but extra peaks appeared in the electrophoretic blank when left in solution for more than a few minutes, presumably due to dye degradation.
3.1 Electrophoretic behavior in SDS-borate buffer
Figure 1 presents the submicellar electropherogram generated from solution of α-lactalbumin that had been labeled at 10−9 M concentration with 10 μM Chromeo P465 in a borax/SDS buffer at 54 °C for 5 min. The blank solution shows a large reagent peak at 60 s, a smaller reagent peak at 100 s, and a broad envelope of peaks between 100 and 130 s. The labeled protein generated an additional broad peak between 107–114 s.
Figure 1.
Electropherogram of 10−9 M α-lactalbumin that had been labeled with Chromeo P465 (top, solid) and the reagent blank (bottom, dashed). Curves offset for clarity.
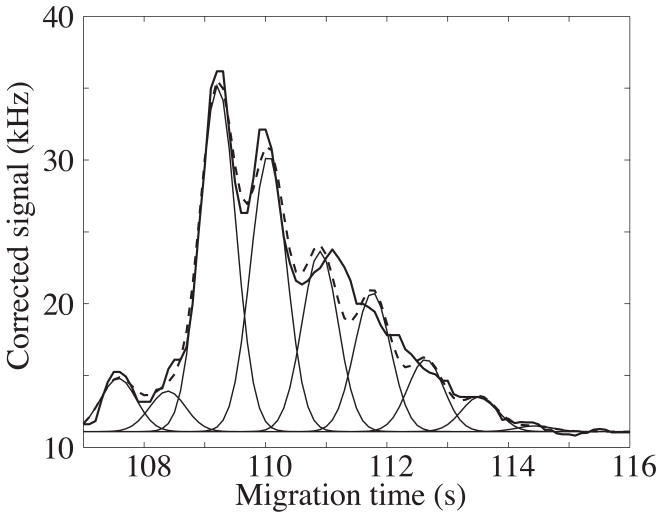
The broad peak appears to consist of a set of poorly resolved components; these components presumably are reaction products of the protein that contain different numbers of labeling reagent. In Figure 2, we fit the envelope with a set of nine Gaussian peaks with equally spaced mobilities. These peaks have an average mobility of ~4 × 10−4 cm2 V−1 s−1 and are separated by ~3 × 10−6 cm2 V−1 s−1.
Figure 2.
Regression analysis of a set of nine Gaussian peaks to the envelope of figure 1. The Gaussian peaks have equal width (0.4 s) and are spaced by a constant mobility shift of 3 × 10−6 cm2 V−1 s−1. The data are the smooth curve and the sum of the Gaussians is the dashed curve.
The broad peak generated less than 1000 theoretical plates. In contrast, the individual components generated ~70 000 plates.
3.3 Calibration curves
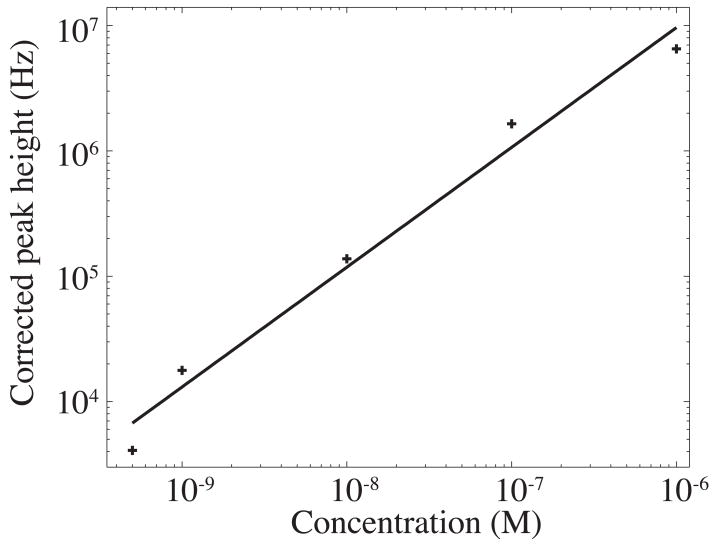
Submicellar electropherograms were generated from five solutions of α-lactalbumin, from 500 pM to 1 μM in concentration that had been reacted with 10 μM Chromeo P465 in borax/SDS buffer 5 min at 54 °C. The signal was corrected to account for the dead time of the avalanche photodiode [15], and the electropherograms were smoothed with a 0.3-s wide Gaussian function. Peak heights of the smoothed data were used to construct a calibration curve. The calibration curve was linear (slope of a log-log plot was 0.96 ± 0.08, r>0.99), figure 3. The 3σ concentration limit of detection for this labeling scheme was 24 pM and the mass detection limit was 15 zmol injected onto the capillary.
Figure 3.
Log of peak height vs. log of concentration of α-lactalbumin labeled with Chromeo P465. Protein was labeled at concentrations ranging from 500 pM to 1 μM. The curve fit to all the data points has a slope of 0.96 ± 0.08 (r = 0.99).
3.3 Separation of a protein mixture
A mixture containing 1.0 μM each of α-lactalbumin (14.2 kDa), α-chymotrypsinogen A (25.7 kDa), and ovalbumin (42.9 kDa) was reacted with 100 μM Chromeo P465 in hydrogencarbonate and diluted to 10 nM in borax/SDS or CHES/Tris/SDS. The three proteins were not baseline resolved with borax/SDS CZE, but decent resolution of these proteins was achieved with CSE (figure 4). The log-log plot of migration time to molecular weight using CSE was linear (r > 0.99).
Figure 4.
Three protein standards labeled with Chromeo P465 and separated by A) CZE and B) CSE. 1.0 μM Ovalbumin (42.9 kDa), α-chymotrypsinogen A (25.7 kDa), and α-lactalbumin (14.2 kDa) were labeled with Chromeo P465 and diluted to 10 nM in either borax/SDS or CHES/Tris/SDS. A. CZE was performed using 2.5 mM borax/3.5 mM SDS on an uncoated capillary. B. CSE was performed using 100 mM CHES/100 mM Tris/3.5 mM SDS/5 %w/v dextran on a capillary coated with UltraTrol LN to suppress electroosmotic flow. The upper trace is the electropherogram of the three-protein mixture; the lower trace is the blank. The asterisks (*) indicate, in order of migration, α-lactalbumin, α-chymotrypsinogen, and ovalbumin.
The blanks achieved with Chromeo P465 are problematic. When the labeling reaction was carried out in hydrogencarbonate and diluted in borax/SDS prior to running, or when dye was diluted in borax/SDS buffer just prior to the labeling reaction, blanks in borax/SDS CZE were clean except for a sharp peak due to the dye. When dye was prepared as a bulk solution in borax/SDS buffer, extra peaks began to appear in the blank over the course of several hours. The unreacted dye peak was very prominent even though the unconjugated dye should have produced very little signal at the excitation wavelengths used here. The blank achieved with CHES/Tris/SDS was less than ideal. In addition to the unreacted dye peak, the blank showed several fluorescent contaminants, some of which co-migrated with labeled proteins, obscuring the signal. The fluorescent species may be products of label degradation or contaminants in the buffer components that are being labeled.
In the following paper in this journal, we investigate the related dye PY503. That dye produced much better electrophoretic performance and was used in a comprehensive two-dimensional capillary electrophoresis separation [12].
Acknowledgments
This work was supported by a grant from the National Institutes of Health (R33CA122900).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wojcik R, Swearingen KE, Dickerson JA, Turner EH, Ramsay LM, Dovichi NJ. J Chromatogr A. 2008 doi: 10.1016/j.chroma.2008.04.042. in press (Part I) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beale SC, Savage JC, Wiesler D, Wiestock SM, Novotny M. Anal Chem. 1988;60:1765. doi: 10.1021/ac00168a025. [DOI] [PubMed] [Google Scholar]
- 3.Hoefelschweiger BK, Duerkop A, Wolfbeis OS. Anal Biochem. 2005;344:122–129. doi: 10.1016/j.ab.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 4.Zhao JY, Waldron KC, Miller J, Zhang JZ, Harke H, Dovichi NJ. J Chromatogr. 1992;608:239–242. doi: 10.1016/0021-9673(92)87129-v. [DOI] [PubMed] [Google Scholar]
- 5.Craig DB, Dovichi NJ. Anal Chem. 1998;70:2493–2494. doi: 10.1021/ac970856v. [DOI] [PubMed] [Google Scholar]
- 6.Richards DP, Stathakis C, Polakowski R, Ahmadzadeh H, Dovichi NJ. J Chromatogr A. 1999;853:21–25. doi: 10.1016/s0021-9673(99)00687-1. [DOI] [PubMed] [Google Scholar]
- 7.Pinto DM, Arriaga EA, Craig D, Angelova J, Sharma N, Ahmadzadeh H, Dovichi NJ, Boulet CA. Anal Chem. 1997;69:3015–3021. doi: 10.1021/ac9611677. [DOI] [PubMed] [Google Scholar]
- 8.Lee IH, Pinto D, Arriaga EA, Zhang ZR, Dovichi NJ. Anal Chem. 1998;70:4546–4548. doi: 10.1021/ac980360t. [DOI] [PubMed] [Google Scholar]
- 9.Zhang ZR, Krylov S, Arriaga EA, Polakowski R, Dovichi NJ. Anal Chem. 2000;72:318–322. doi: 10.1021/ac990694y. [DOI] [PubMed] [Google Scholar]
- 10.Kraly JR, Jones MR, Gomez DG, Dickerson JA, Harwood MM, Eggertson M, Paulson TG, Sanchez CA, Odze R, Feng ZD, Reid BJ, Dovichi NJ. Anal Chem. 2006;78:5977–5986. doi: 10.1021/ac061029+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wetzl BK, Yarmoluk SM, Craig DB, Wolfbeis OS. Angew Chem, Int Ed Engl. 2004;43:5400–5402. doi: 10.1002/anie.200460508. [DOI] [PubMed] [Google Scholar]
- 12.Turner EH, Dickerson JA, Ramsay LM, Wojcik R, Swearingen KE, Dovichi NJ. J Chromatogr A. 2008 doi: 10.1016/j.chroma.2008.04.042. in press (Part III) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng YF, Dovichi NJ. Science. 1988;242:562–4. doi: 10.1126/science.3140381. [DOI] [PubMed] [Google Scholar]
- 14.Wu S, Dovichi NJ. J Chromatogr. 1989;480:141–55. doi: 10.1016/s0021-9673(01)84284-9. [DOI] [PubMed] [Google Scholar]
- 15.Turner EH, Lauterbach K, Pugsley HR, Palmer VR, Dovichi NJ. Anal Chem. 2007;79:778–81. doi: 10.1021/ac061778r. [DOI] [PubMed] [Google Scholar]