Abstract
AIM: To investigate the effects of bombesin (BBS) and neurotensin (NTS) on apoptosis and colitis in an ulcerative colitis model.
METHODS: In this study, a total of 50 rats were divided equally into 5 groups. In the control group, no colitis induction or drug administration was performed. Colitis was induced in all other groups. Following the induction of colitis, BBS, NTS or both were applied to three groups of rats. The remaining group (colitis group) received no treatment. On the 11th d after induction of colitis and drug treatment, blood samples were collected for TNF-α and IL-6 level studies. Malondialdehyde (MDA), carbonyl, myeloperoxidase (MPO) and caspase-3 activities, as well as histopathological findings, evaluated in colonic tissues.
RESULTS: According to the macroscopic and microscopic findings, the study groups treated with BBS, NTS and BBS + NTS showed significantly lower damage and inflammation compared with the colitis group (macroscopic score, 2.1 ± 0.87, 3.7 ± 0.94 and 2.1 ± 0.87 vs 7.3 ± 0.94; microscopic score, 2.0 ± 0.66, 3.3 ± 0.82 and 1.8 ± 0.63 vs 5.2 ± 0.78, P < 0.01). TNF-α and IL-6 levels were increased significantly in all groups compared with the control group. These increases were significantly smaller in the BBS, NTS and BBS + NTS groups compared with the colitis group (TNF-α levels, 169.69 ± 53.56, 245.86 ± 64.85 and 175.54 ± 42.19 vs 556.44 ± 49.82; IL-6 levels, 443.30 ± 53.99, 612.80 ± 70.39 and 396.80 ± 78.43 vs 1505.90 ± 222.23, P < 0.05). The colonic MPO and MDA levels were significantly lower in control, BBS, NTS and BBS + NTS groups than in the colitis group (MPO levels, 24.36 ± 8.10, 40.51 ± 8.67 and 25.83 ± 6.43 vs 161.47 ± 38.24; MDA levels, 4.70 ± 1.41, 6.55 ± 1.12 and 4.51 ± 0.54 vs 15.60 ± 1.88, P < 0.05). Carbonyl content and caspase-3 levels were higher in the colitis and NTS groups than in control, BBS and BBS + NTS groups (carbonyl levels, 553.99 ± 59.58 and 336.26 ± 35.72 vs 209.76 ± 30.92, 219.76 ± 25.77 and 220.34 ± 36.95; caspase-3 levels, 451.70 ± 68.27 and 216.20 ± 28.17 vs 28.60 ± 6.46, 170.50 ± 32.37 and 166.50 ± 30.95, P < 0.05).
CONCLUSION: The results of this study suggest BBS and NTS, through their anti-inflammatory actions, support the maintenance of colonic integrity and merit consideration as potential agents for ameliorating colonic inflammation.
Keywords: Bombesin, Neurotensin, Colitis, Apoptosis
INTRODUCTION
Inflammatory bowel diseases (IBDs), including Crohn’s disease (CD) and ulcerative colitis (UC), are chronic relapsing inflammatory conditions of unknown etiology. The most common symptoms of both UC and CD are diarrhea, which may be profuse and bloody, and abdominal pain[1].
The exact pathogenesis in IBD is poorly understood, but evidence suggests IBD involves interactions between the immune system, genetic susceptibility and the environment[1]. Some studies have analyzed the role of a variety of soluble peptides and other mediators that, once secreted in a co-ordinated fashion in the injured area, are able to restore mucosal integrity[2].
CD affects the full thickness of the wall of the colon or the small intestine, whereas UC primarily affects the mucosa of the colon. Histologically, the affected tissue shows ulceration and the infiltration of inflammatory cells from the circulation[3].
Neuropeptides are small molecules that, in the intestine, modulate several biological processes and modify the activities of cells responsible either for triggering tissue damage or promoting healing[4,5]. The tetradecapeptide bombesin (BBS) was originally isolated from the skin of the amphibian Bombina bombina, and is analogous to mammalian gastrin-releasing peptide[6]. In the gastrointestinal tract, BBS stimulates the secretion of various gut hormones and peptides (for example, gastrin, somatostatin, cholecystokinine, insulin, glucagon, motilin, pancreatic polypeptide and neurotensin), exerts trophic effects on intestinal mucosa and pancreas, and stimulates intestinal motility[7,8]. It has been shown BBS improves intestinal integrity in experimental models of gut barrier dysfunction, like administration of methotrexate and elemental diets[9,10], and that it also has mitogenic effects[11]. Neurotensin (NTS) is a tridecapeptide, found mainly in the brain and gut. Intestinal NTS is produced by a discrete population of endocrine cells scattered throughout the jejeuno-ileal mucosa[12]. The peptide is released into the circulation after food ingestion. Its digestive functions include stimulation of pancreaticobiliary secretions, intestinal blood flow, inhibition of gastric acid secretion and motility, stimulation of colon motility and inhibition of jejuno-ileum motility. It is a potent trophic agent for the small and large intestines[13,14]. NTS has been shown to prevent intestinal atrophy induced by feeding rats an elemental diet, and to enhance intestinal mucosal restoration after small bowel resection[15]; it also has immunoregulatory functions[16].
The intact mucosa and an indigenous intestinal flora are important components of body defenses against luminal pathogenic bacteria. The epithelia of the colon are dynamic and rapidly proliferating tissues that are profoundly affected by changes in luminal contents and injury. The aim of this study was to determine the effects of BBS and NTS on an experimental colitis model, because of their potent trophic, metabolic and mitogenic effects.
MATERIALS AND METHODS
Animals
Male Wistar-albino rats (Erciyes University Hakan Cetinsaya Experimental and Clinical Research Center, Kayseri, Turkey) weighing 225-290 g were fed ad libitum a nutritionally balanced rodent diet and water. They were kept under constant environmental conditions. Water only was provided during the 12 h preceding the experiments. Our institutional ethical committee approved the experimental procedures of this study.
Experimental design
Fifty Wistar-albino rats (28-32 wk) were used in the study. The rats were divided into 5 groups containing 10 rats each: GroupI(Control) = normal animals; Group II (Colitis) = induction of experimental colitis without further treatment; Group III (BBS) = induction of experimental colitis plus administration of bombesin; Group IV (NTS) = induction of experimental colitis plus administration of neurotensin; and Group V (BBS + NTS) = induction of experimental colitis plus administration of bombesin and neurotensin together.
Experimental procedures
Colitis was induced as previously described[17]. Briefly, rats were lightly anesthetized with halothane, and an infant feeding tube (Unoplast A/S DK 330; Hundested, Denmark) fitted onto a blunt 18-gauge needle was inserted rectally. The tip of the tube was placed nearly 8 cm into the colon and 25 mg 2,4,6- trinitrobenzene sulphonic acid (Sigma, St. Louis, Missouri) mixed with 0.25 mL 30% ethanol (TNBS-E) was intracolonically instilled. After instillation, the rats were supported in the supine position until recovery from anesthesia to prevent immediate anal leakage of the instillate. The control group rats received intracolonic saline. For the 10 subsequent days, the animals in groups 3 and 5 were treated daily with BBS (10 μg/kg, subcutaneously, 3 times a day), and those in groups 4 and 5 were treated daily with NTS (300 μg/kg, intraperitoneally, once a day). Rats in control and colitis groups received sterile saline at the same volumes and same times. All injections were given after topical application of an antiseptic solution of povidone iodine 10%.
All rats were fed standard rat chow for 10 d. On the 11th d, all animals were operated on. Rats were anesthetized by intraperitoneal injection of ketamine-HCl (10 mg/kg; Ketalar®, Eczacibasi, Istanbul, Turkey) and xylazin (3 mg/kg; Rompun® 2%, Bayer, Germany). The abdominal skin was cleansed with povidone-iodine after shaving. All procedures were performed under sterile conditions by one surgeon. The abdomen was opened by a midline incision, and a 2-mL blood sample was collected from the portal vein to measure the levels of TNF-α and IL-6. The distal colon was removed for analysis of myeloperoxidase (MPO) activity, carbonyl content (protein oxidation), lipid peroxidation (malondialdehyde [MDA]), caspase-3 activity and histologic examination. At the end of surgical procedure, all rats were sacrificed by overdose ether inhalation.
Peptides preparation
Stock solutions of BBS (Sigma Chemical Co) and NTS (Sigma Chemical Co) were prepared by first dissolving the amount of peptide needed for the study in 1 mL of sterile water containing 0.1% (weight/volume) bovine serum albumin, and then diluted with normal saline containing 1% bovine serum albumin, so that the amounts of BBS and NTS needed for each injection were contained in a volume of 0.1 mL. Aliquots of 1% bovine serum albumin and BBS were stored in plastic tubes at -20°C. To prolong the rate of absorption after each injection, BBS in saline was mixed 1:4 (volume/volume ratio) with 8% (weight/volume) hydrolyzed gelatin (Sigma Chemical Co) before administration. A final volume of 0.5 mL containing 10 μg BBS/kg body weight, was injected subcutaneously 3 times daily[7]. The NTS solution was divided into equal aliquots of 0.1 mL that were stored in glass vials at -20°C. At the time of administration, each aliquot was further diluted with 0.4 mL of sterile saline to a final volume of 0.5 mL, which was given intraperitoneally as a bolus injection containing 300 μg NTS/kg body weight[18].
Determination of serum tumor necrosis factor-alpha and interleukin-6 levels
The levels of serum tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) were determined using specific enzyme-linked immunosorbent assay kits (BioSource Rat TNF-α ELISA and BioSource Rat IL-6 ELISA). The results were expressed as pg/mL.
Assessment of colonic damage
The distal colon was removed, opened longitudinally and fecal material was rinsed with a gentle spray of 0.9% saline. The freshly opened colonic segments were pinned out on a wax block and examined by a pathologist who was blinded to the treatment.
Colon macroscopic score
The extent of mucosal damage was assessed using the colon macroscopic scoring system of Wallace et al (Table 1)[19].
Table 1.
Colon macroscopic scoring system
| Score | Criteria |
| 0 | No damage |
| 1 | Hyperemia |
| 2 | Hyperemia and thickening without ulceration |
| 3 | Ulceration at a single site |
| 4 | Two or more sites of ulceration or inflammation |
| 5 | Ulceration or inflammation extending > 1 cm along the length of colon |
| 6-10 | Damage covering > 2 cm along the lenght of colon, with the score being increased by 1 for each additional centimeter of involvement |
Colon microscopic score
Tissue samles were fixed with 10% formaldehyde solution for 24 h and transferred to 70% ethanol solution. The tissues were embedded in parafin; 4-μm thick sections were made, and histologic examination was performed under a light microscope after hematoxylin and eosin staining. A pathologist who was unaware of the animals’ groupings evaluated the specimens. Tissues were assessed for the presence and activity of colitis as well as the extent of tissue damage using a large number of serial sections. Colonic inflammation was assessed using a modification of the histopathologic grading system of MacPherson and Pfeifer (Table 2)[20].
Table 2.
Histopathologic grading scale of TNBS-E-induced colitis
| Grade of colitis | Microscopic findings |
| 0 | No damage |
| I | Mild mocosal and/or submucosal inflammatory infiltrate and edema: punctuate mucosal erosions often associated with capillary proliferation, muscularis mucosa intact |
| II | Grade I changes involving 50% of the specimen |
| III | Prominent inflammatory infiltrate and edema frequently with deeper areas of ulceration extending through the muscularis mucosa into the submucosa, rare inflammatory cells invading the muscularis propria but without muscle necrosis |
| IV | Grade III changes involving 50% of the specimen |
| V | Extensive ulceration with coagulative necrosis bordered inferiorly by numerous neutrophils and fewer mononuclear cells, necrosis extends deeply into the muscularis propria |
| VI | Grade V changes involving 50% of the specimen |
Myeloperoxidase measurement in colon tissue
After being scored, a sample of distal colon was frozen for subsequent measurement of MPO activity as an index of granulocyte infiltration[19]. The method described by Bradley et al was used to measure MPO activity in colon homogenates[21]. The previously frozen colon tissues were homogenized for 30 s in 4 mL of 20 mmol/L potassium phosphate buffer, pH 7.4, and centrifuged for 30 min at 40 000 × g at 4°C. The pellet was resuspended in 4 mL of 50-mmol/L potassium phosphate buffer, pH 6.0, containing 0.5 g/dL hexadecyltrimethyl ammonium bromide. Samples were sonicated for 90 s at full power, incubated in a 60°C water bath for 2 h and centrifuged. The supernatant (0.1 mL) was added to 2.9 mL of 50-mmol/L potassium phosphate buffer, pH 6.0, containing 0.167 mg/mL dianisidine and 0.0005% hydrogen peroxide. Absorbance of 460 nm visible light (A460) was measured for 3 min. The results were expressed as units of enzyme activity per gram of wet tissue weight (U/g tissue).
Malondialdehyde measurement in colon tissue
One part of the tissue was homogenized in 10 parts of 15 mmol/L KCl for the MDA assay. MDA, which is the end product of fatty acid peroxidation, reacts with thiobarbituric acid (TBA) to form a colored complex. Measurement of MDA content by TBA reactivity is the most widely used methods to assess lipid peroxidation[22]. The principle of the method is based on measurement of the absorbance of the pink color produced by the interaction of TBA with MDA at 530 nm. Values were expressed as nmol/g wet tissue weight.
Determination of carbonyl content
Tissue carbonyl molecule formation was determined to reflect protein oxidation as a biochemical marker of oxidative damage owing to colitis. Protein oxidation was assayed by a modified streptomycin sulfate, 2,4-dinitrophenylhydrazine-based method, and expressed in nmol total carbonyl groups/mg total protein[23].
Caspase-3 proteolytic activity
The caspase-3 activity ratio was calculated using a colorimetric assay (Chemicon International, Temecula, CA). Cells harvested from the different treatment groups were lysed and 200 μg of protein was tested for protease activity by the addition of a caspase-specific substrate peptide, DVED-pnitroaniline. Caspase-3 cleavage of the peptide releases the chromophore p-nitroaniline (p-NA), which was quantitated spectro-photometrically at 405 nm. The level of caspase-3 enzymatic activity in the cell lysate was directly proportional to the extent of the color reaction. The results are expressed as the fold increase of caspase activity in apoptotic cells relative to their respective controls. In background reactions, no DVED-p-NA substrate was added, and the values obtained were subtracted from experimental results before calculating the fold increase[24].
Statistical analysis
The data are presented as means ± SD. One-way analysis of variance (ANOVA) was used to compare the levels of serum TNF-α, IL-6, colon MDA and MPO, and caspase-3, as well as carbonyl content, among the 5 groups. The Tamhane test was used as a post hoc test for multiple comparisons between groups. Non-parametric Kruskal-Wallis tests were used to compare macroscopic and microscopic damage scores. Dunn’s test was used for multiple comparisons between groups. Data were analyzed using Statistical Package for the Social Sciences (SPSS) for Windows (version 13.0). Significance was set at P < 0.05.
RESULTS
Macroscopic colonic damage
Table 3 shows the results of assessment of gross colonic injury. In rats treated with BBS in which a macroscopic response was evident, there was minimal damage to the surface epithelium, and there was mild inflammation of the mucosa, but no transmural inflammation. In the colitis group, the damage comprised broad mucosal ulcers with a surface layer of necrotic slough, accumulation of mesenteric fat and fibrinous adhesions to the bowel. Macroscopic colonic damage score was highest in the colitis group (median; 7.0). This score was significantly lower in BBS and BBS + NTS groups, but higher than that in control rats (P < 0.01). Although the score in rats in NTS group was higher than those in rats in the BBS and BBS + NTS, and lower than that in the colitis group, there was no significant difference (P > 0.05) (Table 3).
Table 3.
Comparison of macroscopic and microscopic colonic damage scores
| Colonic damage score | Group I Control | Group II Colitis | Group III BBS | Group IV NTS | Group V BBS and NTS | P | |
| Macroscopic score | mean ± SD | 0.3 ± 0.48 | 7.3 ± 0.94 | 2.1 ± 0.87 | 3.7 ± 0.94 | 2.1 ± 0.87 | < 0.01 |
| Median (Min-Max) | 0 (0.0-1.0)b | 7 (6.0-9.0)d | 2 (1.0-3.0)b | 4 (2.0-5.0)f | 2 (1.0-3.0)b | ||
| Microscopic score | mean ± SD | 0.4 ± 0.69 | 5.2 ± 0.78 | 2.0 ± 0.66 | 3.3 ± 0.82 | 1.8 ± 0.63 | < 0.01 |
| Median (Min-Max) | 0 (0.0-2.0)b | 5 (4.0-6.0)d | 2 (1.0-3.0)b | 3.5 (2.0-4.0)f | 2 (1.0-3.0)b | ||
Data are expressed as means ± SD and median (min-max).
P < 0.01 vs colitis and NTS;
P < 0.01 vs BBS, NTS and BBS and NTS;
P < 0.01 vs colitis, control, BBS and BBS and NTS. BBS: Bombesin; NTS: Neurotensin.
Microscopic colonic damage
Histopathologic evaluation of the control group was within normal limits. Microscopic examination showed low levels of damage or inflammation in rats treated with BBS (groups III and V), whereas in rats with colitis, there was widespread destruction of the mucosa with transmural infiltration of neutrophils, monocytes and lymphocytes (P < 0.01) (Table 3).
Serum tumor necrosis factor-alpha and interleukin-6 levels
Serum TNF-α and IL-6 levels in the colitis and study groups were significantly increased at d 11 compared with the control group (P < 0.05). This increase was significantly smaller in the BBS, NTS and BBS + NTS groups than in the colitis group (P < 0.05) (Figures 1 and 2).
Figure 1.
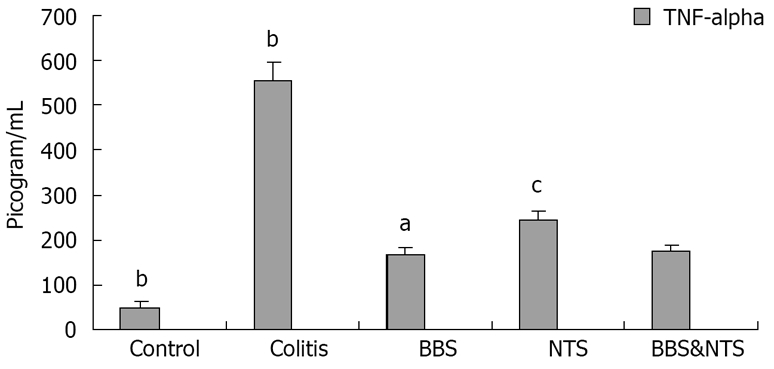
Serum tumor necrosis factor-alpha (TNF-alpha) concentrations in rats. Vertical columns and error bars represent mean ± SD. bP < 0.001 vs BBS, NTS and BBS and NTS; aP < 0.05 vs control, colitis and NTS; cP < 0.05 vs control, colitis, BBS and BBS and NTS. BBS: Bombesin; NTS: Neurotensin.
Figure 2.
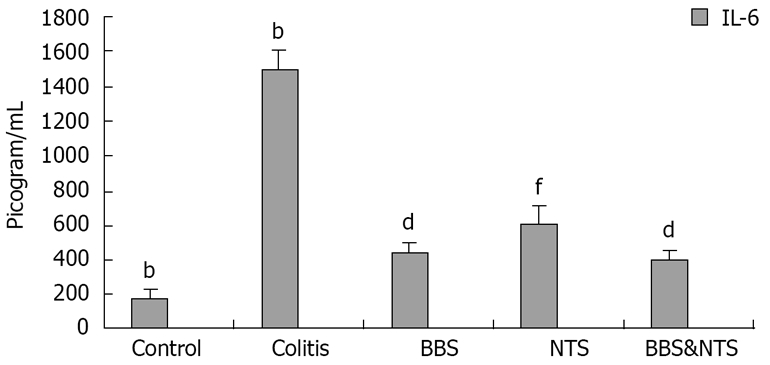
Serum interleukin-6 (IL-6) concentrations in rats. Vertical columns and error bars represent mean ± SD. bP < 0.001 vs BBS, NTS and BBS&NTS; dP < 0.001 vs control, colitis and NTS; fP < 0.001 vs control, colitis and BBS and BBS and NTS. BBS: Bombesin; NTS: Neurotensin.
Myeloperoxidase levels
The colonic tissue MPO levels were higher in the colitis group than in the control, BBS, NTS and BBS + NTS groups (P < 0.001). There were no significant differences between the control and BBS groups (P = 0.27), or between the BBS and BBS + NTS groups (P = 1.00), whereas the MPO levels in the control and BBS + NTS groups were statistically significant different (P = 0.001). The increase in the NTS group was significantly high than the increase in the BBS (P = 0.004) and BBS + NTS groups (P = 0.005) (Figure 3).
Figure 3.
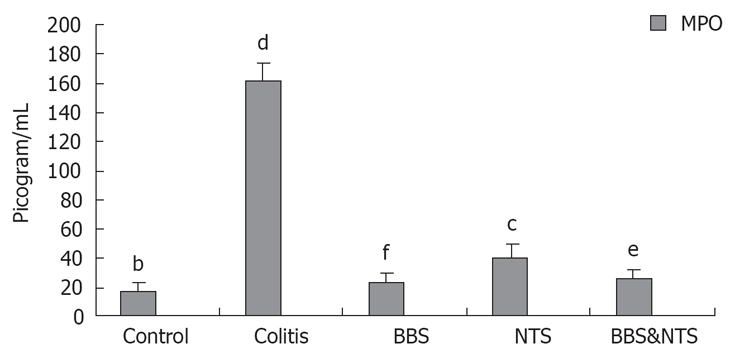
Colon myeloperoxidase (MPO) concentrations in rats. Vertical columns and error bars represent mean ± SD. bP < 0.001 vs colitis and NTS; dP < 0.001 vs control, BBS, NTS and BBS and NTS; fP < 0.001 vs colitis and NTS; cP < 0.05 vs control, colitis, BBS and BBS and NTS; eP < 0.05 vs control, colitis, BBS and NTS. BBS: Bombesin; NTS: Neurotensin.
Malondialdehyde levels
MDA levels were highest in the colitis group, and were significantly higher in the BBS, NTS and BBS + NTS groups compared with the control group (P < 0.01). There was no statistically significant difference between BBS and BBS + NTS groups (P = 1.00), but the MDA levels in these two groups were significantly higher than those in the NTS group (P = 0.047 and P = 0.002) (Figure 4).
Figure 4.
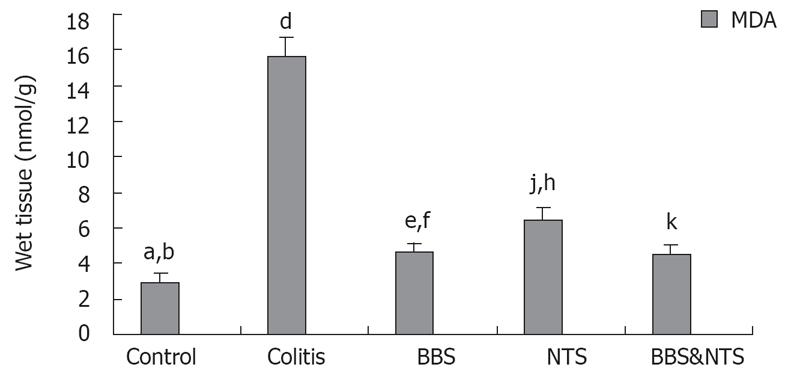
Colon malondialdehyde (MDA) concentrations in rats. Vertical columns and error bars represent mean ± SD. bP < 0.001 vs colitis, NTS and BBS&NTS and aP < 0.05 vs BBS; dP < 0.001 vs control, BBS, NTS and BBS&NTS; fP < 0.001 vs colitis and eP < 0.05 vs control and NTS; hP < 0.001 vs control and colitis, jP < 0.05 vs BBS and BBS and NTS; kP < 0.001 vs control, colitis and NTS. BBS: Bombesin; NTS: Neurotensin.
Carbonyl contents
Carbonyl contents were higher in the colitis and NTS groups compared with the control, BBS and BBS + NTS groups (P < 0.001). The carbonyl contents of the control, BBS and BBS + NTS groups were not significantly different (P > 0.05, range; 0.997-1.00). The carbonyl content of the NTS group was significantly higher than that of the control, BBS and BBS + NTS groups, but lower than that in the colitis group (P < 0.001) (Figure 5).
Figure 5.
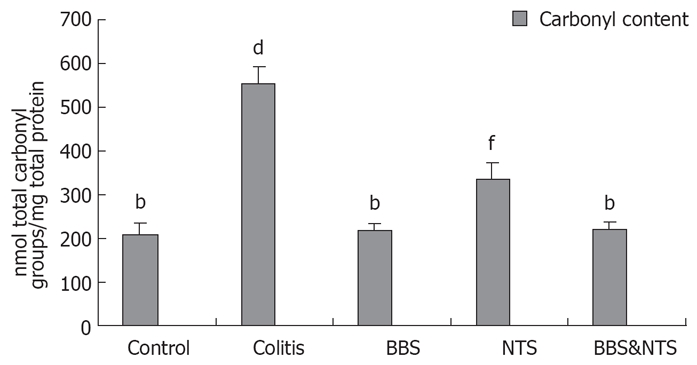
Colonic carbonyl content in rats. Vertical columns and error bars represent mean ± SD. bP < 0.001 vs colitis and NTS; dP < 0.001 vs control, BBS, NTS and BBS&NTS; fP < 0.001 vs control, colitis, BBS and BBS&NTS; BBS: Bombesin; NTS: Neurotensin.
Caspase-3 levels
Colon caspase levels were higher in the colitis and NTS groups than in the control, BBS and BBS + NTS groups (P < 0.05, range; 0.001-0.034). There was no statistically significant difference between the BBS and BBS + NTS groups (P = 1.00), but the increases in caspase-3 levels in these two groups were significantly smaller than those in the control, colitis and NTS groups (P < 0.05, range; 0.001-0.034) (Figure 6).
Figure 6.
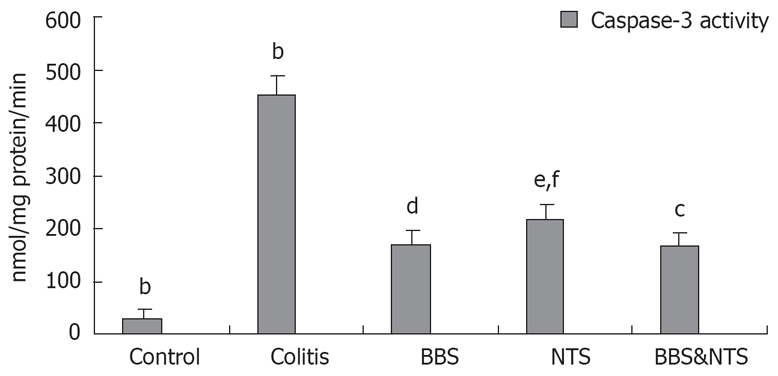
Colonic caspase-3 activity of all groups. Vertical columns and error bars represent mean ± SD. bP < 0.001 vs BBS, NTS and BBS&NTS; dP < 0.001 vs control, colitis and cP < 0.05 vs NTS; fP < 0.001 vs control and colitis, eP < 0.05 vs BBS and BBS and NTS; BBS: Bombesin; NTS: Neurotensin.
DISCUSSION
IBDs are chronic intestinal inflammatory disorders that are considered to be the result of an inappropriate response of the immune system to luminal antigens[25]. Studies over the past several years have shown a variety of soluble peptides, prostaglandins, growth factors, cytokines and chemokines are secreted in a coordinated fashion in the injured area to restore mucosal integrity[26–28]. Although experimental animal models of colitis are of value in eliciting some of the mechanisms underlying IBDs, different models differ in their relative use. Most animal models lack certain characteristics of IBDs and model acute events rather than the chronic features typical of IBDs. TNBS colitis has been well characterized and has clinical, biochemical and pathologic similarities to colonic CD. Animals with TNBS model colitis respond to drugs that have proven useful in IBDs[19]. Therefore, we used TNBS-induced colitis, which has the dual advantage of chronicity and acuteness so often seen in patients with IBD. Some other advantages of this model of colitis are its reasonable reproducibility and its value in assessing the efficacy of therapeutic agents commonly used for the treatment of colitis[19].
BBS and NTS are biologically active neuropeptides that are widely distributed in the central and peripheral nervous systems as well as in the endocrine systems. They have multiple biological effects, such as gastrointestinal motility, mucosal blood flow, cell growth and immunoregulation[29]. Their biological effects may result from direct actions of BBS and NTS, as neurotransmitters, on the target organ, or indirectly through the action of gastrointestinal hormones released in response to these neuropeptides. Furthermore, the nature and amplitude of neuropeptide-mediated effects may be different in normal tissue and in inflammatory disorders, because the levels of receptors and cell populations expressing neuropeptide receptors may be strikingly different[30]. In this study, we aimed to assess the role of BBS and NTS in a model of colitis using TNBS, a well-accepted model for IBD.
Although BBS and NTS improve intestinal barrier function in an experimental colitis model[7,14,18], we don’t know the precise mechanisms underlying these effects. Therefore, we aimed to evaluate the effects of BBS and NTS on lipid peroxidation, protein oxidation and apoptosis in a colitis model. We also aimed to analyse the molecular mechanisms of these effects.
In the colitis group, increased macroscopic and microscopic damage scores revealed severe inflammatory changes. BBS and NTS treatment attenuated the colonic damage, suggesting these agents may have an additional protective role supporting colonic integrity. Although both agents significantly reduced macroscopic and microscopic colonic damage scores, we don’t know if the effects of these two agents are additive or synergistic, and whether these effects are dose-dependent or not. There is no report in the literature about the therapeutic doses of BBS and NTS in experimental colitis models. However, in most studies, both agents were administered at the same doses[7,31]. Brun et al reported the mucosal level of NTS showed a 5.8-fold increase in an experimental colitis model compared with normal mice[4]. On the contrary, in patients with chronic inflammatory disorders, neuropeptide levels have been reported to be low[32]. All of these observations suggest the neuropeptide response depends on the severity of the inflammatory process and the site of the event. Receptor changes may cause these effects. Of the three mammalian bombesin-like receptors, only the type-2 receptor is present in the human intestine. The receptor types present and their localization were not altered in patients with IBD, although there was an obvious decrease in the number of binding sites in the smooth muscle of patients with IBD compared with normal control patients. In the mucosa of patients with IBD, very few binding sites for BBS were seen[33]. This suggests that if the administration of BBS has any effect, it will be observed in the human mucosa, acting via indirect mechanisms such as release of other trophic agents, for example, cholecystokinin, neurotensin.
TNF-α and IL-6 are multifunctional cytokines produced primarily by activated monocytes and macrophages; they play a crucial role in the initiation and continuation of mucosal inflammation and immunity[34,35]. These cytokines are involved in many cell processes including apoptotic cell death, metabolism, inflammation, thrombosis and fibrinolysis[36]. TNF-α and IL-6 induce the production of other cytokines including adhesion molecules and arachidonic acid metabolites, and activation of immune and non-immune cells[37]. Systemic inhibition of soluble TNF-α by infliximab (chimeric anti- TNF-α mAbs) has been reported to induce remission in up to 50% of patients with CD and to significantly improve clinical symptoms in most patients[38]. Worledge et al reported that the administration of TNF-α antibodies effectively treated experimental colitis in rats[39]. Analysis of endoscopic biopsy samples from IBD patients revealed the involved colonic mucosa from patients with active disease contains larger amounts of IL-6 than colonic mucosa from patients in remission[40]. In another study, the administration of anti-IL-6 antibody was shown to ameliorate disease[41]. In our study, TNF-α and IL-6 levels were correlated with the increased level of inflammation in the colitis group. By contrast, TNF-α and IL-6 levels decreased in all study groups, this decrease was more significant in the BBS and BBS + NTS groups. At this point, TNF-α and IL-6 may be promising markers in monitoring the progress of IBD and the response to treatment. Such findings indicate blockade of TNF-α and IL-6 signaling are clinically advantageous in the treatment of IBDs. The results of our study suggest the therapeutic potential of BBS and NTS in human IBDs with decreases TNF-α and IL-6 levels. Assimakopoulos et al reported experimental obstructive jaundice induces regional loss of occludin expression in the intestinal epithelium, which may be a key contributing factor to the disruption of the mucosal barrier. The gut regulatory peptides BBS and NTS prevent this alteration leading to lower portal and systemic endotoxemia and inflammation[31]. These inflammatory responses are characterized by the release of cytokines and other proinflammatory mediators, such as TNF-α, IL-6 and oxygen-free radicals. These agents may produce injurious effects on the structure and function of tight junctions, compromising intestinal epithelial barrier function[35,37,42]. Specifically, it has been demonstrated TNF-α downregulates the human occludin promoter[43]. In addition, endotoxemia and inflammation reduce splanchnic blood flow and disrupt intestinal microcirculation, resulting in hypoxia of enterocytes and energy depletion[44]. Studies in epithelial cells have shown that adenosine triphosphate (ATP) depletion induces the structural perturbation of the tight junction[45]. Increased ATP levels in treatment groups may affect energy balance positively, with improved functional and morphological restoration.
Cell death by apoptosis plays an important role in the regulation of intestinal epithelial homeostasis. Administration of anti-IL-6 and TNF-α antibodies to rats with different forms of experimental colitis leads to decreases in the severity of colitis and the occurrence of apoptotic cells in the inflammed tissues[46]. However, Atreya et al have reported the mechanisms underlying the therapeutic effects of anti-IL-6 antibodies are more profound; they affect the survival of CD4+ T cells mediating inflammation such that the main action of the antibodies is on the cells inducing or producing the cytokines[47]. The potential relevance of modulating T-cell resistance against apoptosis as a therapeutic target in IBD is underlined by Zhou et al, who showed drugs that facilitate apoptosis can be used for the supression of T-cell mediated autoimmune disease[48]. Control animals showed basal levels of apoptotic activity, known as spontaneous apoptosis. TNBS-E-induced colitis resulted in a significant increase in the levels of proinflammatory cytokines and apoptosis, while BBS and NTS reduced the levels of proinflammatory cytokines and the rate of apoptotic death. Potential mechanism for increased apoptosis after TNBS-E induced colitis may implicate the excessive release of IL-6 and TNF-α, activation of cytotoxic T cells. Activated cytotoxic T cells induce death of inflammatory CD4+ T cells via independent and possibly synergistic mechanisms. Assimakopoulos et al reported BBS and NTS signficantly decreased apoptosis in obstructed jaundiced rats, and they speculated this effect occurred by decreasing intestinal oxidative stress, which is an important apoptosis inducer[49]. In addition, it should be taken into consideration that neuropeptide-mediated effects may be modulated by complex interactions with other mediators involved in tissue restitution, such as chemokines[28]. An indirect mechanism may be related to a further reduction of intestinal lipid peroxidation, because oxidative state is involved in the modulation of cell proliferation and death[50]. The anti-apoptotic effect of BBS and NTS may be mediated by a reduction in endotoxemia and amelioration of the subsequent systemic inflammation, which may activate the apoptotic process. Another explanation of their anti-apoptotic action may be through induction of vasodilation[51], which improves intestinal microcirculation, thus preventing hypoxia, energy depletion and activation of apoptotic pathways. The anti-apoptotic effects of BBS and NTS may be mediated by acting on one or more factors. For this reason, the future of therapy for many types of IBDs might lie in combined anti-cytokine, anti-oxidant and anti-apoptotic therapies, rather than treatment with a single agent alone.
It has been proposed that oxygen-derived free radicals are implicated in the pathogenesis of IBDs[52]. Numerous studies showed MDA is formed in increased amounts in intestinal tissue[52,53]. MDA (lipid peroxidation) and carbonyls in cellular proteins (protein oxidation), generated by oxygen free radicals, is believed to be an important cause of damage to cell membranes[23,54]. Previous experimental studies had shown BBS and NTS improved gut barrier functions after chemically induced colitis[13,15]. Similarly, in our study, TNBS-E-induced colitis resulted in increased MDA and carbonyl production in colonic tissue. Additionally, BBS and NTS administration significantly reduced intestinal MDA and carbonyl content in rats with TNBS-E-induced colitis. These results provide evidence of an antioxidant action of BBS and NTS in the intestine in an experimental colitis model. These effects might be related to a reduction in endotoxemia or improvement in intestinal microcirculation induced by BBS and NTS, leading to oxidative metabolism. This antioxidant effect may be attributed to a direct receptor-mediated mechanism. Binding of neuropeptides to their specific receptors is followed by activation of protein kinase[55], which is known to activate intracellular antioxidant enzyme systems. An alternative explanation might be the reduction of endotoxemia, which is associated with the generation of oxygen free radicals via xanthine oxidase-dependent pathways. Moreover, improvement in intestinal microcirculation induced by regulatory neuropeptides prevents hypoxia and energy depletion, leading to oxidative metabolism. Since structural and functional integrity of mitochondria is crucial for the maintenance of cell metabolic homeostasis, this mechanism enhances cellular defenses against oxidant injury.
In our experiments, the inflammatory response caused by TNBS-E is described by an important increase in neutrophil infiltration into the colonic tissue, and the subsequent augmentation of MPO levels. This increase in MPO activity was substantially reduced in rats treated with BBS and NTS. Neutrophils play a crucial role in this regard by producing superoxide anions and a cascade of various reactive species. Activated neutrophils pass out of the circulation and enter the inflammed mucosa and submucosa of the colon during acute inflammation, leading to a large amount of production of reactive oxygen and nitrogen species that can contribute to intestinal injury[56]. MPO catalyzes the formation of such potent cytotoxic oxidants. The reduction in colonic MPO activity, as well as the histological findings of the absence of cellular infiltration following treatment with BBS and NTS, may indicate their antioxidant and anti-inflammatory effects.
The present study has shown TNBS-E-induced ulcerative colitis was associated with macroscopic, microscopic and biochemical changes. Medical treatment of IBDs is principally aimed at inhibiting the production of inflammatory mediators and at modulating the immune system. Treatment with BBS and NTS attenuated colitis as shown by the lower serum levels of TNF-α and IL-6, and the reduced colonic tissue levels of MPO, MDA, carbonyls and caspases. Additionally, these neuropeptides stimulate growth and regeneration of the intestinal mucosa. For these reasons, they have been implicated in the treatment of IBDs. However, further studies are needed to explain the cellular mechanism underlying the trophic and regenerative effects of BBS and NTS.
COMMENTS
Background
The exact pathogenesis in inflammatory bowel diseases is poorly understood, but some studies have analyzed the role of a variety of soluble peptides and other mediators that, once secreted in a co-ordinated fashion in the injured area, are able to restore mucosal integrity.
Research frontiers
The epithelium of the colon consists of dynamic and rapidly proliferating cells that are profoundly affected by changes in luminal contents and injury. Previous experimental studies have shown intrarectal instillation of 2,4,6- trinitrobenzene sulphonic acid (Sigma, St. Louis, Missouri) mixed with 30% ethanol (TNBS-E) promotes morphologic alterations in the colonic mucosa indicative of atrophy, increases apoptosis and induces intestinal oxidative stress. Treatment with bombesin and neurotensin attenuated colitis, as shown by the lower serum levels of tumor necrosis factor-alpha and interleukin-6, and the reduced colonic tissue levels of myeloperoxidase, malondialdehyde, carbonyls and caspases.
Innovations and breakthroughs
This study demonstrates the gut trophic peptides bombesin and neurotensin improve TNBS-E-induced ulcerative colitis. This effect related to a regulatory effect of peptides on epithelial cell production and apoptosis, and reduction of intestinal oxidative stress. Although the results of laboratory experiments are not readily applicable to the clinical situation, these findings offer further insight into the pathophysiology of gut barrier functions and suggest an important biologic effect of regulatory peptides.
Applications
The results from this experimental study confirm the protective effect of bombesin and neurotensine on TNBS-E-induced colitis and apoptosis. This encourages us to apply these agents in treating inflammatory bowel diseases in the future clinical trials.
Terminology
Bombesin and neurotensin exert a wide spectrum of biologic actions on gastrointestinal tissues, influencing intestinal motility, blood flow, secretion, absorption and immune responses. These agents are small molecules that, in the intestine, modulate several biological processes and modify the activity of cells responsible for promoting healing.
Peer review
This is a well-written manuscript. The authors describe the effect of ameliorative effects of bombesin (BBS) and neurotensin (NTS) on trinitrobenzene sulphonic acid-induced colitis.
Supported by Grant (SBAG-105S338) from the Scientific and Technical Research Council of Turkey (TUBITAK)
Peer reviewers: Tsuneo Kitamura, Associate Professor, Department of Gastroenterology, Juntendo University Urayasu Hospital, Juntendo University School of Medicine, 2-1-1 Tomioka, Urayasu-shi, Chiba 279-0021, Japan; Yoshiharu Motoo, MD, PhD, FACP, FACG, Professor and Chairman, Department of Medical Oncology, Kanazawa Medical University,1-1 Daigaku, Uchinada, Ishikawa 920-0293, Japan
S- Editor Zhu LH L- Editor Kerr C E- Editor Lu W
References
- 1.MacDonald TT, Monteleone G, Pender SL. Recent developments in the immunology of inflammatory bowel disease. Scand J Immunol. 2000;51:2–9. doi: 10.1046/j.1365-3083.2000.00658.x. [DOI] [PubMed] [Google Scholar]
- 2.Podolsky DK. Mucosal immunity and inflammation. V. Innate mechanisms of mucosal defense and repair: the best offense is a good defense. Am J Physiol. 1999;277:G495–G499. doi: 10.1152/ajpgi.1999.277.3.G495. [DOI] [PubMed] [Google Scholar]
- 3.Riddell RH. Pathology of idiopathic inflammatory bowel disease. In: Kirsner JB, editor. Inflammatory bowel disease, 5th ed. Philadelphia: WB Saunders; 2000. pp. 427–450. [Google Scholar]
- 4.Brun P, Mastrotto C, Beggiao E, Stefani A, Barzon L, Sturniolo GC, Palu G, Castagliuolo I. Neuropeptide neurotensin stimulates intestinal wound healing following chronic intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2005;288:G621–G629. doi: 10.1152/ajpgi.00140.2004. [DOI] [PubMed] [Google Scholar]
- 5.Scheffer M, Beiter T, Becker HD, Hunt TK. Neuropeptides: mediators of inflammation and tissue repair? Arch Surg. 1998;133:1107–1116. doi: 10.1001/archsurg.133.10.1107. [DOI] [PubMed] [Google Scholar]
- 6.Anastasi A, Erspamer V, Bucci M. Isolation and amino acid sequences of alytesin and bombesin, two analogous active tetradecapeptides from the skin of European discoglossid frogs. Arch Biochem Biophys. 1972;148:443–446. doi: 10.1016/0003-9861(72)90162-2. [DOI] [PubMed] [Google Scholar]
- 7.Chu KU, Evers BM, Ishizuka J, Townsend CM Jr, Thompson JC. Role of bombesin on gut mucosal growth. Ann Surg. 1995;222:94–100. doi: 10.1097/00000658-199507000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu KU, Higashide S, Evers BM, Ishizuka J, Townsend CM Jr, Thompson JC. Bombesin stimulates mucosal growth in jejunal and ileal Thiry-Vella fistulas. Ann Surg. 1995;221:602–609; discussion 609-611. doi: 10.1097/00000658-199505000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu KU, Higashide S, Evers BM, Rajaraman S, Ishizuka J, Townsend CM Jr, Thompson JC. Bombesin improves survival from methotrexate-induced enterocolitis. Ann Surg. 1994;220:570–576; discussion 576-577. doi: 10.1097/00000658-199410000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evers BM, Izukura M, Townsend CM Jr, Uchida T, Thompson JC. Differential effects of gut hormones on pancreatic and intestinal growth during administration of an elemental diet. Ann Surg. 1990;211:630–636; discussion 636-638. [PMC free article] [PubMed] [Google Scholar]
- 11.Micheletti R, Grider JR, Makhlouf GM. Identification of bombesin receptors on isolated muscle cells from human intestine. Regul Pept. 1988;21:219–226. doi: 10.1016/0167-0115(88)90004-3. [DOI] [PubMed] [Google Scholar]
- 12.Polak JM, Sullivan SN, Bloom SR, Buchan AM, Facer P, Brown MR, Pearse AG. Specific localisation of neurotensin to the N cell in human intestine by radioimmunoassay and immunocytochemistry. Nature. 1977;270:183–184. doi: 10.1038/270183a0. [DOI] [PubMed] [Google Scholar]
- 13.Chung DH, Evers BM, Shimoda I, Townsend CM Jr, Rajaraman S, Thompson JC. Effect of neurotensin on gut mucosal growth in rats with jejunal and ileal Thiry-Vella fistulas. Gastroenterology. 1992;103:1254–1259. doi: 10.1016/0016-5085(92)91512-3. [DOI] [PubMed] [Google Scholar]
- 14.Evers BM, Izukura M, Chung DH, Parekh D, Yoshinaga K, Greeley GH Jr, Uchida T, Townsend CM Jr, Thompson JC. Neurotensin stimulates growth of colonic mucosa in young and aged rats. Gastroenterology. 1992;103:86–91. doi: 10.1016/0016-5085(92)91099-p. [DOI] [PubMed] [Google Scholar]
- 15.Olsen PS, Pedersen JH, Poulsen SS, Yamashita Y, Kirkegaard P. Neurotensin-like immunoreactivity after intestinal resection in the rat. Gut. 1987;28:1107–1111. doi: 10.1136/gut.28.9.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Rio M, De la Fuente M. Chemoattractant capacity of bombesin, gastrin-releasing peptide and neuromedin C is mediated through PKC activation in murine peritoneal leukocytes. Regul Pept. 1994;49:185–193. doi: 10.1016/0167-0115(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 17.Yano Y, Yao H, Aoyagi K, Kawakubo K, Nakamura S, Doi K, Ibayashi S, Fujishima M. Photochemically induced colonic ischaemic lesions: a new model of ischaemic colitis in rats. Gut. 1997;41:354–357. doi: 10.1136/gut.41.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vagianos C, Karatzas T, Scopa CD, Panagopoulos C, Tsoni I, Spiliopoulou I, Kalfarentzos F. Neurotensin reduces microbial translocation and improves intestinal mucosa integrity after abdominal radiation. Eur Surg Res. 1992;24:77–83. doi: 10.1159/000129191. [DOI] [PubMed] [Google Scholar]
- 19.Wallace JL, MacNaughton WK, Morris GP, Beck PL. Inhibition of leukotriene synthesis markedly accelerates healing in a rat model of inflammatory bowel disease. Gastroenterology. 1989;96:29–36. doi: 10.1016/0016-5085(89)90760-9. [DOI] [PubMed] [Google Scholar]
- 20.MacPherson BR, Pfeiffer CJ. Experimental production of diffuse colitis in rats. Digestion. 1978;17:135–150. doi: 10.1159/000198104. [DOI] [PubMed] [Google Scholar]
- 21.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 22.Rungby J, Flyvbjerg A, Andersen HB, Nyborg K. Lipid peroxidation in early experimental diabetes in rats: effects of diabetes and insulin. Acta Endocrinol (Copenh) 1992;126:378–380. doi: 10.1530/acta.0.1260378. [DOI] [PubMed] [Google Scholar]
- 23.Reznick ZA, Packer L. Oxidative damate to proteins: spectrophotometric method for carbony assay. In: Abelson NJ and Simon IM., editor. Methods in Enzymology. v: Academic Press; 1994. pp. 357–363. [DOI] [PubMed] [Google Scholar]
- 24.Avivi-Green C, Madar Z, Schwartz B. Pectin-enriched diet affects distribution and expression of apoptosis-cascade proteins in colonic crypts of dimethylhydrazine-treated rats. Int J Mol Med. 2000;6:689–698. doi: 10.3892/ijmm.6.6.689. [DOI] [PubMed] [Google Scholar]
- 25.Scaldaferri F, Fiocchi C. Inflammatory bowel disease: progress and current concepts of etiopathogenesis. J Dig Dis. 2007;8:171–178. doi: 10.1111/j.1751-2980.2007.00310.x. [DOI] [PubMed] [Google Scholar]
- 26.Podolsky DK. Mucosal immunity and inflammation. V. Innate mechanisms of mucosal defense and repair: the best offense is a good defense. Am J Physiol. 1999;277:G495–G499. doi: 10.1152/ajpgi.1999.277.3.G495. [DOI] [PubMed] [Google Scholar]
- 27.Procaccino F, Reinshagen M, Hoffmann P, Zeeh JM, Lakshmanan J, McRoberts JA, Patel A, French S, Eysselein VE. Protective effect of epidermal growth factor in an experimental model of colitis in rats. Gastroenterology. 1994;107:12–17. doi: 10.1016/0016-5085(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 28.Kucuk C, Sozuer E, Gursoy S, Canoz O, Artis T, Akcan A, Akyildiz H, Muhtaroglu S. Treatment with Met-RANTES decreases bacterial translocation in experimental colitis. Am J Surg. 2006;191:77–83. doi: 10.1016/j.amjsurg.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Walsh JH. Gastrointestinal hormones. In: Physiology of the Gastrointestinal Tract (2nd ed.), Johnson LR., editors. New York: Raven; 1987. p. 181–253. [Google Scholar]
- 30.Mantyh CR, Gates TS, Zimmerman RP, Welton ML, Passaro EP Jr, Vigna SR, Maggio JE, Kruger L, Mantyh PW. Receptor binding sites for substance P, but not substance K or neuromedin K, are expressed in high concentrations by arterioles, venules, and lymph nodules in surgical specimens obtained from patients with ulcerative colitis and Crohn disease. Proc Natl Acad Sci USA. 1988;85:3235–3239. doi: 10.1073/pnas.85.9.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Assimakopoulos SF, Scopa CD, Charonis A, Spiliopoulou I, Georgiou C, Nikolopoulou V, Vagianos CE. Experimental obstructive jaundice disrupts intestinal mucosal barrier by altering occludin expression: beneficial effect of bombesin and neurotensin. J Am Coll Surg. 2004;198:748–757. doi: 10.1016/j.jamcollsurg.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 32.Sharkey KA. Substance P and calcitonin gene-related peptide (CGRP) in gastrointestinal inflammation. Ann N Y Acad Sci. 1992;664:425–442. doi: 10.1111/j.1749-6632.1992.tb39781.x. [DOI] [PubMed] [Google Scholar]
- 33.ter Beek WP, Muller ES, Van Hogezand RA, Biemond I, Lamers CB. Gastrin releasing peptide receptor expression is decreased in patients with Crohn's disease but not in ulcerative colitis. J Clin Pathol. 2004;57:1047–1051. doi: 10.1136/jcp.2003.014993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tracey KJ, Cerami A. Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu Rev Med. 1994;45:491–503. doi: 10.1146/annurev.med.45.1.491. [DOI] [PubMed] [Google Scholar]
- 35.Mitsuyama K, Sata M, Tanikawa K. Significance of interleukin-6 in patients with inflammatory bowel disease. Gastroenterol Jpn. 1991;26:20–28. doi: 10.1007/BF02779504. [DOI] [PubMed] [Google Scholar]
- 36.Nilsen EM, Johansen FE, Jahnsen FL, Lundin KE, Scholz T, Brandtzaeg P, Haraldsen G. Cytokine profiles of cultured microvascular endothelial cells from the human intestine. Gut. 1998;42:635–642. doi: 10.1136/gut.42.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bobin-Dubigeon C, Collin X, Grimaud N, Robert JM, Le Baut G, Petit JY. Effects of tumour necrosis factor-alpha synthesis inhibitors on rat trinitrobenzene sulphonic acid-induced chronic colitis. Eur J Pharmacol. 2001;431:103–110. doi: 10.1016/s0014-2999(01)01410-8. [DOI] [PubMed] [Google Scholar]
- 38.Nikolaus S, Raedler A, Kuhbacker T, Sfikas N, Folsch UR, Schreiber S. Mechanisms in failure of infliximab for Crohn's disease. Lancet. 2000;356:1475–1479. doi: 10.1016/s0140-6736(00)02871-3. [DOI] [PubMed] [Google Scholar]
- 39.Worledge KL, Godiska R, Barrett TA, Kink JA. Oral administration of avian tumor necrosis factor antibodies effectively treats experimental colitis in rats. Dig Dis Sci. 2000;45:2298–2305. doi: 10.1023/a:1005554900286. [DOI] [PubMed] [Google Scholar]
- 40.Mitsuyama K, Sasaki E, Toyonaga A, Ikeda H, Tsuruta O, Irie A, Arima N, Oriishi T, Harada K, Fujisaki K. Colonic mucosal interleukin-6 in inflammatory bowel disease. Digestion. 1991;50:104–111. doi: 10.1159/000200747. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto M, Yoshizaki K, Kishimoto T, Ito H. IL-6 is required for the development of Th1 cell-mediated murine colitis. J Immunol. 2000;164:4878–4882. doi: 10.4049/jimmunol.164.9.4878. [DOI] [PubMed] [Google Scholar]
- 42.Xu DZ, Lu Q, Deitch EA. Nitric oxide directly impairs intestinal barrier function. Shock. 2002;17:139–145. doi: 10.1097/00024382-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 43.Mankertz J, Tavalali S, Schmitz H, Mankertz A, Riecken EO, Fromm M, Schulzke JD. Expression from the human occludin promoter is affected by tumor necrosis factor alpha and interferon gamma. J Cell Sci. 2000;113(Pt 11):2085–2090. doi: 10.1242/jcs.113.11.2085. [DOI] [PubMed] [Google Scholar]
- 44.Nakajima Y, Baudry N, Duranteau J, Vicaut E. Microcirculation in intestinal villi: a comparison between hemorrhagic and endotoxin shock. Am J Respir Crit Care Med. 2001;164:1526–1530. doi: 10.1164/ajrccm.164.8.2009065. [DOI] [PubMed] [Google Scholar]
- 45.Tsukamoto T, Nigam SK. Role of tyrosine phosphorylation in the reassembly of occludin and other tight junction proteins. Am J Physiol. 1999;276:F737–F750. doi: 10.1152/ajprenal.1999.276.5.F737. [DOI] [PubMed] [Google Scholar]
- 46.Pritchard DM, Watson AJ. Apoptosis and gastrointestinal pharmacology. Pharmacol Ther. 1996;72:149–169. doi: 10.1016/s0163-7258(96)00102-7. [DOI] [PubMed] [Google Scholar]
- 47.Atreya R, Mudter J, Finotto S, Müllberg J, Jostock T, Wirtz S, Schütz M, Bartsch B, Holtmann M, Becker C, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583–588. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 48.Zhou T, Song L, Yang P, Wang Z, Lui D, Jope RS. Bisindolylmaleimide VIII facilitates Fas-mediated apoptosis and inhibits T cell-mediated autoimmune diseases. Nat Med. 1999;5:42–48. doi: 10.1038/4723. [DOI] [PubMed] [Google Scholar]
- 49.Assimakopoulos SF, Scopa CD, Zervoudakis G, Mylonas PG, Georgiou C, Nikolopoulou V, Vagianos CE. Bombesin and neurotensin reduce endotoxemia, intestinal oxidative stress, and apoptosis in experimental obstructive jaundice. Ann Surg. 2005;241:159–167. doi: 10.1097/01.sla.0000149306.35717.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 51.Harper SL, Barrowman JA, Kvietys PR, Granger DN. Effect of neurotensin on intestinal capillary permeability and blood flow. Am J Physiol. 1984;247:G161–G166. doi: 10.1152/ajpgi.1984.247.2.G161. [DOI] [PubMed] [Google Scholar]
- 52.Simmonds NJ, Rampton DS. Inflammatory bowel disease--a radical view. Gut. 1993;34:865–868. doi: 10.1136/gut.34.7.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogawa Y, Kanatsu K, Iino T, Kato S, Jeong YI, Shibata N, Takada K, Takeuchi K. Protection against dextran sulfate sodium-induced colitis by microspheres of ellagic acid in rats. Life Sci. 2002;71:827–839. doi: 10.1016/s0024-3205(02)01737-x. [DOI] [PubMed] [Google Scholar]
- 54.Reiter RJ, Tan DX, Manchester LC, Qi W. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: a review of the evidence. Cell Biochem Biophys. 2001;34:237–256. doi: 10.1385/CBB:34:2:237. [DOI] [PubMed] [Google Scholar]
- 55.Rozengurt E. Signal transduction pathways in the mitogenic response to G protein-coupled neuropeptide receptor agonists. J Cell Physiol. 1998;177:507–517. doi: 10.1002/(SICI)1097-4652(199812)177:4<507::AID-JCP2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 56.Abreu MT. The pathogenesis of inflammatory bowel disease: translational implications for clinicians. Curr Gastroenterol Rep. 2002;4:481–489. doi: 10.1007/s11894-002-0024-0. [DOI] [PubMed] [Google Scholar]


