Abstract
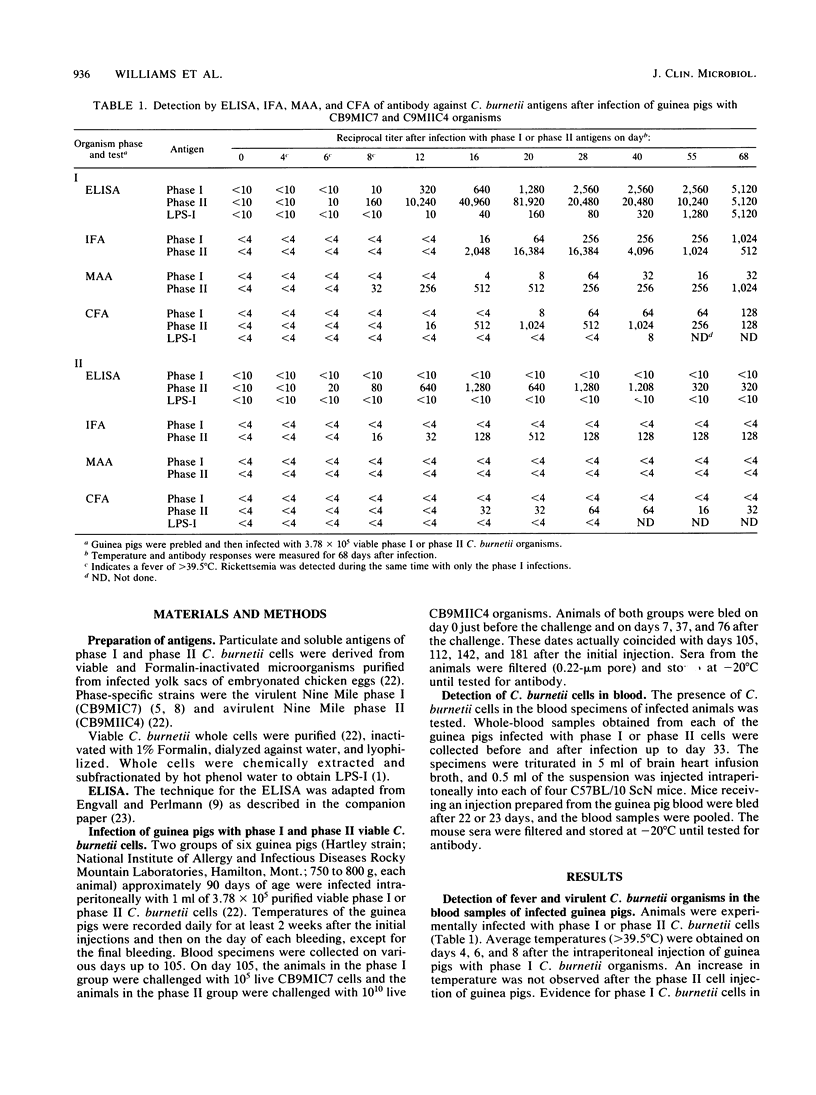
The response of guinea pigs experimentally infected with Coxiella burnetii organisms, the etiologic agents of Q fever, was obtained by the measurement of fever, circulating infectious C. burnetii cells, and anti-C. burnetii antibodies. The detection of antibodies by the enzyme-linked immunosorbent assay (ELISA) and traditional methods against phase I whole cells, phase II whole cells, and phase I lipopolysaccharide (LPS-I) (a virulence marker for phase I cells) antigens in the serum samples of infected animals revealed marked differences between intrastrain phase variants. Animals infected with the phase I Nine Mile strain produced a concomitant increase in temperature, circulating infectious C. burnetii cells, and antibodies against phase II cells, phase I cells, and LPS-I. At 15 weeks, a challenge of phase I-infected animals with viable phase I cells resulted in anamnestic antibody responses to phase I cells and LPS-I but not to phase II cells. Infection of animals with the phase II Nine Mile strain produced antibodies against only phase II cells. The challenge of phase II-infected animals at 15 weeks with viable phase II cells resulted in anamnestic antibody responses to phase I and phase II cells but not to LPS-I. Suppression of anti-phase II responses by the phase I challenge was apparent with only the ELISA, because the immunofluorescence, microagglutination, and complement fixation assays were insensitive to these changes. The sensitivity and specificity of the ELISA with whole-cell and the LPS-I antigens in the detection of phase-specific antibody revealed that avirulent phase II cells induced an immune response to phase I antigenic epitopes. Although the avirulent phase II cells were rapidly cleared by the host immune responses, they were sufficiently infective to induce antibody responses to both phase variants. Thus, in the occurrence of Q fever, any conventional serological technique that uses only phase II antigens may not provide a true incidence of naturally acquired infection with both phase I and II C. burnetii organisms.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano K., Williams J. C. Chemical and immunological characterization of lipopolysaccharides from phase I and phase II Coxiella burnetii. J Bacteriol. 1984 Dec;160(3):994–1002. doi: 10.1128/jb.160.3.994-1002.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher M. S., Berman M. A., Ruppanner R. Initial clinical and immunologic evaluation of a new phase I Q fever vaccine and skin test in humans. J Infect Dis. 1983 Aug;148(2):214–222. doi: 10.1093/infdis/148.2.214. [DOI] [PubMed] [Google Scholar]
- Ascher M. S., Horwith G. S., Thornton M. F., Greenwood J. R., Berman M. A. A rapid immunofluorescent procedure for serodiagnosis of Q fever in mice, guinea pigs, sheep, and humans. Diagn Immunol. 1983;1(1):33–38. [PubMed] [Google Scholar]
- Crăcea E., Constantinescu S., Dumitrescu A., Stefănescu M., Szegli G. ELISA in the Q fever diagnosis. Arch Roum Pathol Exp Microbiol. 1983 Oct-Dec;42(4):283–288. [PubMed] [Google Scholar]
- Damrow T. A., Williams J. C., Waag D. M. Suppression of in vitro lymphocyte proliferation in C57BL/10 ScN mice vaccinated with phase I Coxiella burnetii. Infect Immun. 1985 Jan;47(1):149–156. doi: 10.1128/iai.47.1.149-156.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Fiset P., Ormsbee R. A., Silberman R., Peacock M., Spielman S. H. A microagglutination technique for detection and measurement of rickettsial antibodies. Acta Virol. 1969 Jan;13(1):60–66. [PubMed] [Google Scholar]
- Fiset P., Wike D. A., Pickens E. G., Ormsbee R. A. An antigenic comparison of strains of Coxiella burneti. Acta Virol. 1971 Mar;15(2):161–166. [PubMed] [Google Scholar]
- LACKMAN D. B., GILDA G., PHILIP R. N. APPLICATION OF THE RADIOISOTOPE PRECIPITATION TEST TO THE STUDY OF Q FEVER IN MAN. Health Lab Sci. 1964 Jan;1:21–28. [PubMed] [Google Scholar]
- LUOTO L. A capillary-tube test for antibody against Coxiella burnetii in human, guinea pig, and sheep sera. J Immunol. 1956 Nov;77(5):294–298. [PubMed] [Google Scholar]
- ORMSBEE R. A., BELL E. J., LACKMAN D. B., TALLENT G. THE INFLUENCE OF PHASE ON THE PROTECTIVE POTENCY OF Q FEVER VACCINE. J Immunol. 1964 Mar;92:404–412. [PubMed] [Google Scholar]
- Peacock M. G., Philip R. N., Williams J. C., Faulkner R. S. Serological evaluation of O fever in humans: enhanced phase I titers of immunoglobulins G and A are diagnostic for Q fever endocarditis. Infect Immun. 1983 Sep;41(3):1089–1098. doi: 10.1128/iai.41.3.1089-1098.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOKER M. G., FISET P. Phase variation of the Nine Mile and other strains of Rickettsia burneti. Can J Microbiol. 1956 May;2(3):310–321. doi: 10.1139/m56-036. [DOI] [PubMed] [Google Scholar]
- Schmeer N. Enzymimmuntest (ELISA) zum Nachweis von IgG1-, IgG2- und IgM-Antikörpern bei der Q-Fieber-Infektion des Rindes. Zentralbl Bakteriol Mikrobiol Hyg A. 1985 Feb;259(1):20–34. [PubMed] [Google Scholar]
- Schramek S., Brezina R., Urvölgyi J. A new method of preparing diagnostic Q fever antigen. Acta Virol. 1972 Nov;16(6):487–492. [PubMed] [Google Scholar]
- TABERT G. G., LACKMAN D. B. THE RADIOISOTOPE PRECIPITATION TEST FOR STUDY OF Q FEVER ANTIBODIES IN HUMAN AND ANIMAL SERA. J Immunol. 1965 Jun;94:959–965. [PubMed] [Google Scholar]
- Urvölgyi J. Preparing and staining of Coxiella burnetii natural phase II antigen for the microagglutination reaction. Acta Virol. 1976 Jun;20(3):238–242. [PubMed] [Google Scholar]
- Williams J. C., Peacock M. G., McCaul T. F. Immunological and biological characterization of Coxiella burnetii, phases I and II, separated from host components. Infect Immun. 1981 May;32(2):840–851. doi: 10.1128/iai.32.2.840-851.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Thomas L. A., Peacock M. G. Identification of phase-specific antigenic fractions of Coxiella burnetti by enzyme-linked immunosorbent assay. J Clin Microbiol. 1986 Dec;24(6):929–934. doi: 10.1128/jcm.24.6.929-934.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying P. K., Deng R. L., Liu G. Z. Experimental study of enzyme-linked immunosorbent assay (ELISA) for detection of Q fever antibody. Acta Acad Med Wuhan. 1983;3(3):137–142. doi: 10.1007/BF02856646. [DOI] [PubMed] [Google Scholar]


