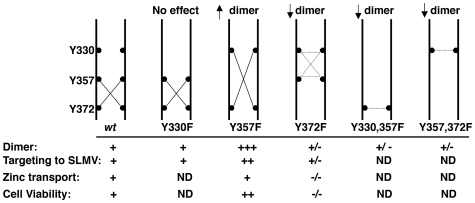
Figure 9. Model of ZnT3 dimer formation deduced from single and double tyrosine mutations.
ZnT3 carboxy-terminal domains of two adjacent ZnT3 molecules are depicted by two vertical parallel black lines. Tyrosine residues and their position are illustrated in black circles. Lines connecting black circles depict covalent tyrosine bonds. Single and double mutant indicate that the predominant dimer is formed between Y372-Y357. Sorting to SLMVs and zinc transport was evaluated in the gain-of-function ZnT3Y357F and the loss-of-function ZnT3Y372F mutants. Increased dimerization of the ZnT3Y357F mutant increased its targeting to SLMVs without affecting its zinc transport capacity, measured by zinquin staining, but increase resistance to zinc toxicity. In contrast, mutation of Y372 decreases SLMV sorting, completely prevents zinc transport and increase cell toxicity to zinc. +/− denotes reduce respect to wild type and ND experiment not done.