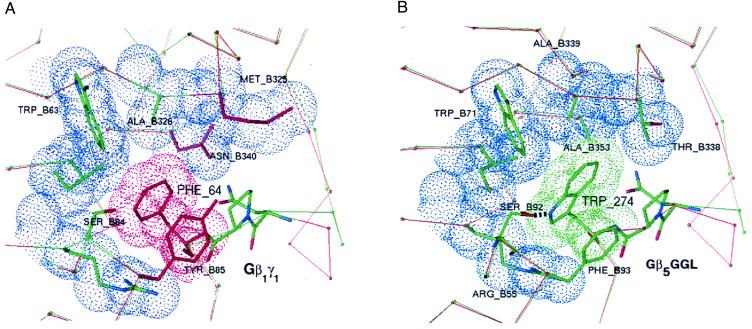
Figure 5.
Specificity-determining residues at the interface of Gβ1 and Gγ1 compared with equivalent regions of modeled Gβ5 and the GGL domain of RGS11. Highlighted in blue are van der Waals surfaces of Gβ1 contacting Phe-64 of Gγ1 (A) or similar contacts between Gβ5 and Trp-274 of the RGS11 GGL domain (B). Residues colored red in the Gβ1/Gγ1 structure differ from equivalent residues in the Gβ5/GGL model. Except for the conserved tripeptide motif (NPF or NPW), thin red and green lines trace the Cα-backbone of Gβ1/Gγ1 and Gβ5/GGL, respectively. Just before the conserved tripeptide motif, the Cα-traces diverge because of the insertion of a single residue in Gγ1 relative to the GGL domain.