Table 1.
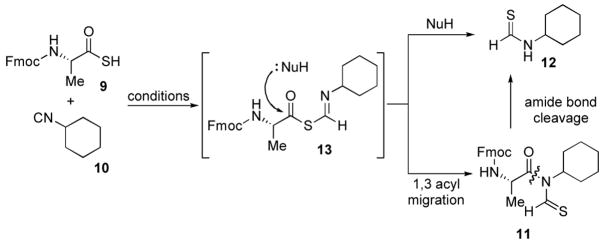 | ||
|---|---|---|
| Entry | Conditionsa | Yieldb |
| 1 | DMF, 40 ºC, 2 h | 21%c |
| 2 | DMF, microwave, 60 ºC,25 min | 22% |
| 3 | Ether, r.t., 6 h | 50% |
| 4 | CHCl3, r.t., 6 h | 53% d |
1.0 equiv. of thioacid and 2.0 equiv. of isonitrile were used for all reactions.
isolated yields for 11.
35% of the cyclohexyl thioformamide was isolated.
71% based on the recovery of thioacid.
