Abstract
A panel of new cytomegalovirus (CMV) assays for use on the Architect instrument has been developed, including a CMV avidity assay based on a new technology. The purpose of this study was to compare the performance characteristics of the fully automated CMV immunoglobulin M (IgM), IgG, and IgG avidity tests on the Architect instrument with those of other available assays. A total of 503 consecutive fresh patient serum specimens (routine serum specimens) and 96 serum specimens from 33 pregnant women with a recent CMV primary infection (seroconversion serum specimens) were tested for CMV IgM and IgG by the Architect (Abbott), Vidas (BioMérieux), and Enzygnost (Siemens) assays. The seroconversion sera and 100 preselected serum specimens IgM negative and IgG positive by the AxSYM assay were also tested by the IgG avidity tests on the Architect and Vidas instruments. The relative agreements for CMV IgM determination with routine sera between the Architect assay and the Vidas, Enzygnost, and AxSYM assays were 97%, 94%, and 93%, respectively, for the CMV IgM tests and 99%, 98%, and 98%, respectively, for the CMV IgG tests. The specificities of the CMV IgG avidity test were 98% for the Architect assay and 76% for the Vidas assay. No high CMV IgG avidity test results were found within the first 3 months after seroconversion by either of those assays. The correlation between the results of the newly developed CMV IgM and IgG tests on the Architect instrument with the Vidas and Enzygnost assays was excellent (≥94%). The CMV IgG avidity test reliably excluded patients with recent infections and showed an excellent specificity (98%).
Human cytomegalovirus (CMV) is the most common cause of congenital infection. Primary infections occur in 0.15 to 2.0% of all pregnancies. The in utero transmission of CMV can take place during primary maternal infection or during nonprimary infection (reactivation and reinfection) of seropositive mothers, but the transmission rate to the fetus is much higher for nonimmune mothers (up to 40%) than for immune mothers (0 to 1%) (16). Fowler et al. showed that naturally acquired immunity results in a 69% reduction in the risk of congenital CMV infection in future pregnancies (2).
Screening of pregnant women for CMV antibodies is a controversial issue and is not supported by international guidelines. Opponents of screening for CMV antibodies during pregnancy suggest that no clearly effective intervention is available. In addition, no tests can reliably predict which infected fetuses will have serious sequelae. The prognostic value of determination of the viral load in amniotic fluid is still a matter of debate and has the disadvantage of its invasive character (3, 5, 7, 10, 13, 15). On the other hand, many parents wish to have an antenatal diagnosis of intrauterine infections, and gynecologists generally offer screening for CMV antibodies. Prenatal screening certainly has advantages, such as the fact that the use of precautionary hygienic measures can be suggested to CMV-seronegative pregnant women. The knowledge of a primary CMV infection in a pregnant woman can lead to closer follow-up of the fetus by ultrasound and nuclear magnetic resonance.
For the diagnosis of a primary CMV infection during pregnancy, it is of utmost importance that we have available reliable, noninvasive tests. CMV-specific immunoglobulin M (IgM) is produced during primary infection, but it is also detectable during reactivation and reinfection (9). A test that enables the discrimination of a primary infection from a nonprimary infection is important for counseling of the parents on the risk of congenital infection. CMV IgG avidity testing has been shown to be useful for distinguishing primary and nonprimary infections (1, 4, 6, 9). It measures the binding affinity of IgG antibodies. At the onset of infection, IgG antibodies of low avidity are produced. Over time, maturation of the antibody occurs, resulting in an increased binding affinity and, thus, a higher avidity. A diagnostic algorithm for CMV serology screening of pregnant women on the basis of CMV IgM, IgG, and IgG avidity testing was proposed by Munro et al. (12).
Recently, a panel of new CMV assays for use on the Architect instrument has been developed, including a CMV IgG avidity assay. It is the first platform that enables the complete automation of avidity testing. While conventional avidity assays remove low-avidity antibodies by the use of a chaotropic agent, in tests with the Architect instrument, high-avidity antibodies are removed by neutralization with liquid antigen and low-avidity antibodies are detected directly. The purpose of the study described here was to evaluate the performance characteristics of the Architect CMV IgM, IgG, and IgG avidity assay and to compare the results with those of the CMV assays available from bioMérieux and Siemens.
MATERIALS AND METHODS
Human serum samples.
The human serum samples used had been sent to the laboratories of the University Hospitals Leuven and the University Hospital St. Luc (Brussels, Belgium) for routine diagnostic evaluation.
(i) Routine specimens.
A total of 503 consecutive fresh serum specimens submitted for CMV serology testing on the AxSYM instrument (Abbott Laboratories, Abbott Park, IL) were used to evaluate the correlation between the results of the Architect (Abbott), AxSYM (Abbott), Vidas (bioMérieux), and Enzygnost assays (Siemens). One hundred consecutive specimens CMV IgM negative and IgG positive by the AxSYM assay were selected to evaluate the specificity of the IgG avidity tests.
(ii) Seroconversion sera.
Ninety-four selected frozen serum specimens from 31 pregnant women with a recent primary CMV infection were used to evaluate the IgG avidity tests. For 25 of these 31 women, a recent seroconversion was known to have occurred over the previous 2 weeks to 4 months. The number of serum specimens from each patient ranged from one to nine.
Determination of CMV IgM, CMV IgG, and CMV IgG avidity.
All routine specimens were tested by commercially available assays from three different manufacturers, namely, the AxSYM CMV IgM and IgG assay, the Architect CMV IgM and IgG assay, the Vidas CMV IgM and IgG assay, and the Enzygnost anti-CMV IgM and IgG assay. All assays with the exception of the Enzygnost assay are fully automated; the Enzygnost assay was performed on a BEPIII instrument (Siemens). The main characteristics of the assays used, including the interpretation of the results, are summarized in Table 1. One hundred consecutively selected serum samples CMV IgM negative and IgG positive by the AxSYM assay were additionally tested by the Architect and Vidas CMV IgG avidity tests.
TABLE 1.
Characteristics of CMV assays
| Assay | Manufacturer | Antigen | Test type and procedurea | Unit | Interpretation of results |
|---|---|---|---|---|---|
| Architect CMV IgG | Abbott | Viral lysate | CMIA, two steps, indirect anti-IgG detection | AU/ml | Nonreactive, <6.0; reactive, ≥6.0 |
| AxSYM CMV IgG | Abbott | Viral lysate | MEIA, two steps, indirect anti-IgG detection | AU/ml | Negative, <5; positive, ≥15 |
| Enzygnost CMV IgG | Siemens | Purified antigen | EIA, two steps, indirect anti-IgG detection | Absorbance value | Negative <0.100; positive, >0.200; equivocal, 0.100-0.200 |
| Vidas CMV IgG | BioMérieux | Viral lysate | ELFA, two steps, indirect anti-IgG detection | AU/ml | Negative, <4; positive, >6; equivocal, ≥4-≤6 |
| Architect CMV IgM | Abbott | Viral lysate and recombinant antigen | CMIA, two steps, indirect anti-IgM detection | Index | Nonreactive, <0.85; reactive, ≥1.00; equivocal, 0.85-0.99 |
| AxSYM CMV IgM | Abbott | Recombinant antigen | MEIA, two steps, indirect anti-IgM detection | Index | Negative, <0.400; positive, ≥0.500; equivocal, 0.400-0.499 |
| Enzygnost CMV IgM | Siemens | Purified antigen | EIA, two steps, indirect anti-IgM detection | Absorbance value | Negative, <0.100; positive, >0.200; equivocal, 0.100-0.200 |
| Vidas CMV IgM | BioMérieux | Viral lysate | ELFA, two steps, indirect anti-IgM detection | Index | Negative, <0.70; positive, ≥0.90; equivocal, 0.70-<0.89 |
| Architect CMV IgG avidity | Abbott | Viral lysate | CMIA, two assays with and without liquid CMV antigen to neutralize high-avidity CMV antibodies | % Avidity | Low avidity, <50.0%; high avidity, >60.0%; equivocal, 50.0-59.9% |
| Vidas CMV IgG avidity | BioMérieux | Viral lysate | ELFA, two assays with and without 6 M urea to dissociate low-avidity antibodies | Avidity index | Low avidity, <0.2; high avidity, >0.8; equivocal, 0.2-<0.8 |
CMIA, chemiluminescent microparticle immunoassay; MEIA, microparticle enzyme immunoassay; EIA, enzyme immunoassay; ELFA, enzyme-linked fluorescent assay.
Selected specimens from pregnant women with a recent primary CMV infection were tested by the Architect CMV IgM, IgG, and IgG avidity assays and the Vidas CMV IgM, IgG, and IgG avidity assays, as well as by the Enzygnost anti-CMV IgG and IgM assays.
Frozen specimens were centrifuged before they were tested. All tests were performed and the results were interpreted according to the instructions of the manufacturer. On the Architect instrument, CMV IgG antibodies with avidities of <50.0% were considered to be low-avidity antibodies, IgG antibodies with avidities of between 50.0% and 59.9% were considered to have results in the gray zone, and IgG antibodies with avidities of ≥60.0% were considered to be high-avidity antibodies. On the Vidas instrument, CMV IgG antibodies with avidities of ≤20.0% were considered to be low-avidity antibodies, IgG antibodies with avidities of between 20% and 80% were considered to represent results in the gray zone, and IgG antibodies with avidities of ≥80.0% were considered to be high-avidity antibodies.
Performance evaluation.
The relative agreement between the results of two assays was calculated as follows: (number of samples with concordant results/total number of samples tested by both assays) × 100. Results in the gray zone were considered positive in this calculation.
The correlation between the results for positive serum samples was calculated as follows: (number of positive samples with concordant results/number of samples that tested positive by either one or both assays) × 100.
Statistics.
The differences in CMV IgG avidity in sera with a low CMV IgG concentration and sera with a high CMV IgG concentration were analyzed by the t test with Analyze-it software.
RESULTS
Of the 503 routine serum samples tested, 44 (8.7%) were reactive by the Architect CMV IgM assay, with 2 samples having results in the gray zone and 42 samples having positive results. Thirty (71.4%) of the 42 positive serum specimens were positive by all assays tested. Only 1 serum sample (0.2%) was reactive (gray zone result) exclusively on the Architect instrument, whereas 15 serum samples (3.0%) were reactive only on the AxSYM instrument. The correlation between the results of the different assays for CMV IgM determination is given in Table 2. The relative rates of agreement between the results of the Architect assay and those of the Vidas, Enzygnost, and AxSYM CMV IgM tests were 97%, 94%, and 93%, respectively. Results in the gray zone were considered positive in these calculations. The levels of correlation between the results for positive sera between the Architect assay and the Vidas, Enzygnost, and AxSYM CMV IgM tests were 79%, 72%, and 55%, respectively. Results in the gray zone were not taken into account for this calculation.
TABLE 2.
Correlation between results of Architect assay and those of Vidas, Enzygnost, and AxSYM CMV IgM assays with 503 routine samples
| Architect assay result | No. of samples with the indicated result by the following assay:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Vidas assay
|
Enzygnost assay
|
AxSYM assay
|
|||||||
| Negative | Gray zone | Positive | Negative | Gray zone | Positive | Negative | Gray zone | Positive | |
| Negative | 457 | 3 | 0 | 432 | 25 | 3 | 434 | 10 | 16 |
| Gray zone | 2 | 0 | 0 | 1 | 0 | 1 | 2 | 0 | 0 |
| Positive | 8 | 3 | 31 | 1 | 8 | 33 | 7 | 3 | 32 |
Of the 503 serum samples tested, 241 were reactive by the Architect CMV IgG assay (prevalence of CMV IgG antibodies, 48%). Twelve serum samples (2.4%) tested negative by the AxSYM assay but were positive by the Architect assay. One serum sample was positive only by the Architect assay. This sample was from a lung transplant recipient who had tested positive by the AxSYM CMV IgG test in the past. One serum sample was positive only by the Vidas assay. That serum sample was from a heart transplant recipient who had tested negative by the AxSYM assay in the past. All cultures of specimens (from different body sites) taken from this patient were negative for CMV. The numbers of serum specimens reactive for CMV IgG by the different assays are given in Table 3. The relative rates of agreement between the Architect assay and the Vidas, Enzygnost, and AxSYM CMV IgG tests were 99%, 98%, and 98%, respectively. Results in the gray zone were considered positive in these calculations. The levels of correlation between the results for positive sera between the Architect assay and the Vidas, Enzygnost, and AxSYM CMV IgG tests were 96%, 96%, and 95%, respectively.
TABLE 3.
Correlation between results of Architect assay and those of Vidas, Enzygnost. and AxSYM CMV IgG assays with 503 routine samples
| Architect assay result | No. of samples with the indicated result by the following assay:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Vidas assay
|
Enzygnost assay
|
AxSYM assay
|
||||||
| Negative | Gray zone | Positive | Negative | Gray zone | Positive | Negative | Positive | |
| Negative | 260 | 1 | 1 | 253 | 8 | 1 | 262 | 0 |
| Positive | 2 | 6 | 233 | 2 | 12 | 227 | 12 | 229 |
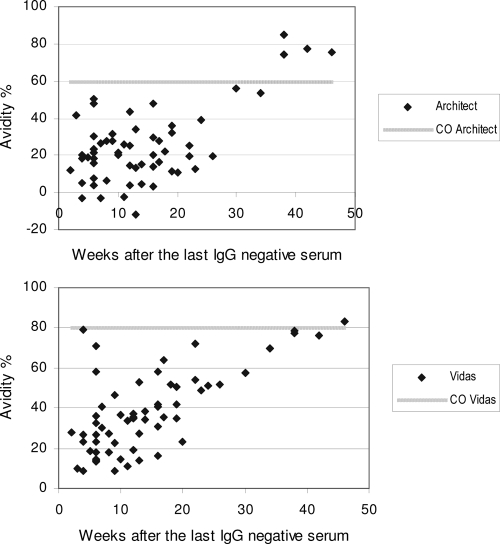
Figure 1 presents the percent avidity for all seroconversion sera in relation to the time of sampling after collection of the last CMV IgG-negative serum sample. No high-avidity CMV IgG test results were found within the first 3 months after seroconversion by the Architect or Vidas CMV IgG avidity assay. High avidity was attained earlier on the Architect instrument than on the Vidas instrument for seven patients. In three patients, no clear gradual increase in avidity was observed after seroconversion, although at least four serum samples were drawn over a period of at least 3 months after seroconversion. On the Vidas instrument, a small increase in IgG avidity was seen in these patients, whereas a decrease in the avidity value was sometimes even seen on the Architect instrument (data not shown).
FIG. 1.
CMV IgG avidity results in relation to the time of sampling after collection of the last CMV IgG-negative serum sample in pregnant woman (CO, cutoff value for high avidity).
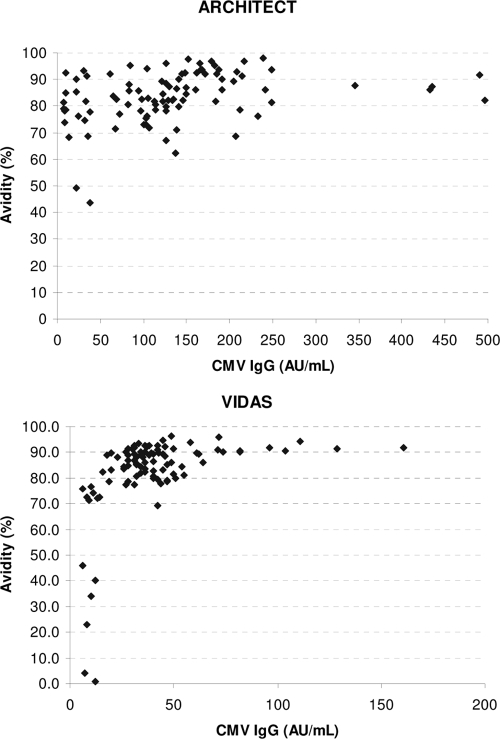
One hundred preselected serum samples IgG positive and IgM negative by the AxSYM assay were tested by the CMV IgG avidity test on the Architect and Vidas instruments for evaluation of the specificity of the CMV IgG avidity tests. On the Architect instrument, the avidity was high (≥60%) for 98 serum specimens (specificity of CMV IgG avidity, 98%). The two samples with low-avidity results (44% and 49%, respectively) were from immunosuppressed patients after transplantation. On the Vidas instrument, the avidity was high (≥80%) for 76% of the serum specimens (specificity of CMV IgG avidity, 76%). Very low avidities (1 and 4%) were found for two serum specimens on the Vidas instrument. The CMV IgG concentration was low in these two serum specimens (12 and 7 arbitrary units [AU]/ml). The avidities according to the CMV IgG concentration for the Architect and Vidas instruments are given in Fig. 2. The avidity was statistically lower (P < 0.001) for sera with a low IgG concentration (<25 AU/ml; mean avidity, 60.2%) than for sera with a higher IgG concentration (>25 AU/ml; mean avidity, 85.9%) on the Vidas instrument (t test). This phenomenon was not observed on the Architect instrument (mean avidities, 78.1% and 84.4%, respectively; P = 0.091).
FIG. 2.
CMV IgG avidity according to the CMV IgG concentration for the Architect and Vidas instruments tested with 100 preselected serum samples IgG positive and IgM negative by the AxSYM assay.
DISCUSSION
The performance characteristics of the new CMV assays developed for performance on the Architect instrument, including a CMV IgG avidity test, were evaluated in this study with 503 routine serum specimens and 94 seroconversion serum specimens. The correlation between the results of the CMV IgM and IgG tests on the Architect instrument with the results of the Vidas and Enzygnost assays was excellent (≥94%). The CMV IgG avidity test reliably excluded patients with recent infections and showed an excellent specificity (98%).
In the context of the diagnosis of a congenital CMV infection, it is important to have a reliable CMV IgM assay. There is no “gold standard” for the determination of CMV IgM antibodies. Therefore, in several studies, one of the tests is chosen to be the standard or the majority of test results in agreement by different methods is considered the true test result (8, 11). Of course, such an approach has important limitations. We evaluated the correlation between the results of the Architect assay and those of the other comparator assays. The fact that only one serum sample among the routine serum samples tested was positive exclusively on the Architect instrument supports the good specificity of this test, which is probably better than the specificity of the CMV IgM test on the AxSYM instrument (15 serum samples were positive exclusively by the CMV IgM test on the AxSYM instrument) and similar to that of the Vidas test. The use of a more specific CMV IgM assay for the screening of pregnant women decreases the need for additional avidity testing of the CMV IgG antibodies. The discrepancy in the results for the CMV IgM-reactive sera on the different systems may partly be due to the different capacities of the different systems to detect only the primary CMV IgM and not the CMV IgM produced during reactivation of the virus. Before the widespread availability of molecular methods for the monitoring of immunocompromised patients for CMV disease, serological methods were used for this indication. In this context, CMV IgM assays were designed for the sensitive detection of IgM in the context of reactivation. Nowadays, molecular methods have been shown to be superior to serological tests for the diagnosis of CMV infection in the immunocompromised patient population, and serological methods are mainly used for the diagnosis of CMV infection in immunocompetent patients and pregnant woman. A positive point about the Architect CMV IgM assay is that it had a less populated gray zone than the two other assays. With the Enzygnost assay, 6.6% of the results were in the gray zone when routine samples were tested. Results in the gray zone are difficult to interpret by clinicians and often result in the testing of follow-up sera. This additional testing may cause anxiety in the parents and results in additional costs.
IgM antibodies are also produced during reactivation and reinfection or are even due to polyclonal stimulation. CMV IgM antibodies can also persist at a low level for up to 39 weeks, depending upon the individual patient and the sensitivity of the IgM assay used (14). All these factors complicate the interpretation of a positive CMV IgM test result tremendously. A test which enables the distinction between primary and nonprimary CMV infection is highly needed. The CMV IgG avidity assay has been shown to have this capacity. The combination of a CMV IgM test with a low CMV IgG avidity improves the specificity of the diagnosis of a primary infection. Patients with antibodies with a high avidity can be reassured that they have a low risk for in utero transmission. From our results, it is clear that there is a large variation in the avidity of IgG in relation to the time of sampling after the collection of the last CMV IgG-negative serum sample in pregnant women. This is partly due to the fact that the exact timing of infection is not known and cannot be taken into account. Important interindividual differences in the rates of maturation of IgG antibodies are also observed, however. In a minority of the patients, no clear maturation was seen during the first 3 months after seroconversion. It is not clear if this phenomenon is more common in pregnant woman than nonpregnant women and is an interesting point for further research. With the Architect assay, a decrease in avidity results was sometimes even seen. This is possibly due to the fact that different dilution protocols are automatically used, depending on the IgG concentration. Small differences in IgG concentration may sometimes result in the application of a different dilution protocol, and this may possibly cause irregularities in the evolution of the IgG avidity results for particular patients. The company does not position the avidity test as a quantitative test but positions it as a qualitative test. Further studies are needed to evaluate the increase in the avidity values on the Architect instrument in patients after seroconversion. This is relevant, as in a woman with low-avidity results, a follow-up serum sample is often drawn and a significant increase in avidity is used to confirm a recent primary infection.
An important part (24%) of the serum samples from patients with past infection tested for CMV IgG avidity did not reach the cutoff for high avidity on the Vidas instrument. The consequence of this is that about one-quarter of pregnant women with a past infection cannot be reassured that they have a low risk for in utero transmission when the Vidas assay is used. From our results, it seems that lowering of the cutoff level on the Vidas instrument is not a solution, as some rather high-avidity results were sometimes obtained early after seroconversion. To our surprise, we noticed a correlation between the IgG concentration and the avidity on the Vidas instrument. Low-avidity results for sera from patients with past infections were nearly exclusively seen in sera with a low IgG concentration. On the Vidas instrument, a statistically significant difference in avidity results was observed between sera with a low IgG concentration and sera with a higher IgG concentration. Although the avidity of the IgG antibodies can be determined in sera with an IgG concentration greater than or equal to 6 AU/ml, according to the guidelines of the manufacturer, it seems that the test is not reliable with sera with IgG concentrations less than 20 to 25 AU/ml.
In conclusion, the correlation of the results between the newly developed CMV IgM and IgG tests on the Architect instrument with the results of several well-established comparator assays was excellent and was the highest with the Vidas test. The CMV IgG avidity test reliably excluded patients with recent infections and showed an excellent specificity.
Acknowledgments
The study was funded by Abbott Diagnostics.
Footnotes
Published ahead of print on 1 April 2009.
REFERENCES
- 1.Bodeus, M., S. Feyder, and P. Goubau. 1998. Avidity of IgG antibodies distinguishes primary from non-primary cytomegalovirus infection in pregnant women. Clin. Diagn. Virol. 99-16. [DOI] [PubMed] [Google Scholar]
- 2.Fowler, K. B., S. Stagno, and R. F. Pass. 2004. Interval between births and risk of congenital cytomegalovirus infection. Clin. Infect. Dis. 381035-1037. [DOI] [PubMed] [Google Scholar]
- 3.Gouarin, S., E. Gault, A. Vabret, D. Cointe, F. Rozenberg, L. Grangeot-Keros, P. Barjot, A. Garbarg-Chenon, P. Lebon, and F. Freymuth. 2002. Real-time PCR quantification of human cytomegalovirus DNA in amniotic fluid samples from mothers with primary infection. J. Clin. Microbiol. 401767-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grangeot-Keros, L., M. J. Mayaux, P. Lebon, F. Freymuth, G. Eugene, R. Stricker, and E. Dussaix. 1997. Value of cytomegalovirus (CMV) IgG avidity index for the diagnosis of primary CMV infection in pregnant women. J. Infect. Dis. 175944-946. [DOI] [PubMed] [Google Scholar]
- 5.Guerra, B., T. Lazzarotto, S. Quarta, M. Lanari, L. Bovicelli, A. Nicolosi, and M. P. Landini. 2000. Prenatal diagnosis of symptomatic congenital cytomegalovirus infection. Am. J. Obstet. Gynecol. 183476-482. [DOI] [PubMed] [Google Scholar]
- 6.Kanengisser-Pines, B., Y. Hazan, G. Pines, and Z. Appelman. 2009. High cytomegalovirus IgG avidity is a reliable indicator of past infection in patients with positive IgM detected during the first trimester of pregnancy. J. Perinat. Med. 3715-18. [DOI] [PubMed] [Google Scholar]
- 7.Lazzarotto, T., L. Gabrielli, M. P. Foschini, M. Lanari, B. Guerra, V. Eusebi, and M. P. Landini. 2003. Congenital cytomegalovirus infection in twin pregnancies: viral load in the amniotic fluid and pregnancy outcome. Pediatrics 112e153-e157. [DOI] [PubMed] [Google Scholar]
- 8.Lazzarotto, T., C. Galli, R. Pulvirenti, R. Rescaldani, R. Vezzo, A. La Gioia, C. Martinelli, S. La Rocca, G. Agresti, L. Grillner, M. Nordin, M. van Ranst, B. Combs, G. T. Maine, and M. P. Landini. 2001. Evaluation of the Abbott AxSYM cytomegalovirus (CMV) immunoglobulin M (IgM) assay in conjunction with other CMV IgM tests and a CMV IgG avidity assay. Clin. Diagn. Lab. Immunol. 8196-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lazzarotto, T., P. Spezzacatena, P. Pradelli, D. A. Abate, S. Varani, and M. P. Landini. 1997. Avidity of immunoglobulin G directed against human cytomegalovirus during primary and secondary infections in immunocompetent and immunocompromised subjects. Clin. Diagn. Lab. Immunol. 4469-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazzarotto, T., S. Varani, B. Guerra, A. Nicolosi, M. Lanari, and M. P. Landini. 2000. Prenatal indicators of congenital cytomegalovirus infection. J. Pediatr. 13790-95. [DOI] [PubMed] [Google Scholar]
- 11.Maine, G. T., R. Stricker, M. Schuler, J. Spesard, S. Brojanac, B. Iriarte, K. Herwig, T. Gramins, B. Combs, J. Wise, H. Simmons, T. Gram, J. Lonze, D. Ruzicki, B. Byrne, J. D. Clifton, L. E. Chovan, D. Wachta, C. Holas, D. Wang, T. Wilson, S. Tomazic-Allen, M. A. Clements, G. L. Wright, Jr., T. Lazzarotto, A. Ripalti, and M. P. Landini. 2000. Development and clinical evaluation of a recombinant-antigen-based cytomegalovirus immunoglobulin M automated immunoassay using the Abbott AxSYM analyzer. J. Clin. Microbiol. 381476-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munro, S. C., B. Hall, L. R. Whybin, L. Leader, P. Robertson, G. T. Maine, and W. D. Rawlinson. 2005. Diagnosis of and screening for cytomegalovirus infection in pregnant women. J. Clin. Microbiol. 434713-4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Picone, O., J. M. Costa, M. Leruez-Ville, P. Ernault, M. Olivi, and Y. Ville. 2004. Cytomegalovirus (CMV) glycoprotein B genotype and CMV DNA load in the amniotic fluid of infected fetuses. Prenat. Diagn. 241001-1006. [DOI] [PubMed] [Google Scholar]
- 14.Revello, M. G., and G. Gerna. 2002. Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clin. Microbiol. Rev. 15680-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Revello, M. G., M. Zavattoni, M. Furione, F. Baldanti, and G. Gerna. 1999. Quantification of human cytomegalovirus DNA in amniotic fluid of mothers of congenitally infected fetuses. J. Clin. Microbiol. 373350-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stagno, S., and R. J. Whitley. 1985. Herpesvirus infections of pregnancy. Part I. Cytomegalovirus and Epstein-Barr virus infections. N. Engl. J. Med. 3131270-1274. [DOI] [PubMed] [Google Scholar]