Abstract
We describe the first two cases of Tsukamurella keratitis, presented as eye pain with or without blurring of vision. One case was associated with trichiasis and the other with contact lens wear. The two isolates were identified as T. tyrosinosolvens and T. pulmonis, respectively, by phenotypic characterization and 16S rRNA sequencing.
CASE REPORTS
Case 1.
An 87-year-old Chinese woman with hypertension and hyperlipidemia presented with left eye pain and blurring of vision for 1 day in August 2007. She had a history of bilateral upper lid trichiasis and had one previous episode of right eye corneal abrasion with no infection in May 2007. Examination of the left eye revealed a 1-mm by 0.5-mm epithelial defect with infiltrates in the inferotemporal quadrant of the cornea near the limbus associated with pannus. Culture of corneal scrapings recovered a gram-positive bacillus. The patient recovered after 10 days of treatment with 0.5% levofloxacin ophthalmic drops.
Case 2.
A 25-year-old Chinese man with an unremarkable past medical history presented with left eye redness and pain in August 2008. He had a long history of contact lens wear with long daily duration of wear, usually more than 16 h per day. Examination of the left eye showed 360° vascular ingrowth at the corneal periphery and a 1.5-mm abscess at the inferonasal quadrant of the cornea near the limbus. There was mild anterior chamber reaction. No hypopyon was noted. Culture of corneal scrapings recovered a gram-positive bacillus and Serratia species. Cultures of swabs from the patient's contact lens case and of the contact lens solution recovered Serratia species and Pseudomonas species. The patient was treated with 0.5% levofloxacin ophthalmic drops and ofloxacin ointment for 3 weeks, and 0.3% gentamicin ophthalmic drops were added after 1 week in view of the contact lens case and solution culture results. The corneal abscess and anterior chamber reaction subsequently resolved, leaving a 1.5-mm corneal scar.
Microbiological data.
All clinical data were collected prospectively. Clinical specimens were collected and handled according to standard protocols (12). All suspect colonies were identified by standard conventional biochemical methods (12). Biochemical reactions in the API 20C AUX and API 50 CH systems (bioMérieux, Lyon, France) were also used for identifying the two strains of gram-positive bacilli, with Tsukamurella pulmonis and Tsukamurella tyrosinosolvens used as controls, as we previously described (24). On day 2 postincubation, a gram-positive aerobic nonsporulating bacillus was recovered from the cultures of the corneal scrapings from both patients. Both isolates were acid fast according to a modified acid-fast stain. Both isolates grew on blood agar as yellow, rough, irregular, dry, but easily emulsified, 2-mm diameter colonies after 48 h of incubation at 37°C in an aerobic environment with 5% CO2. For comparisons with those of other Tsukamurella species, the major phenotypic characteristics of the two isolates are summarized in Table 1. The phenotypic characteristics of the isolate recovered from patient 1 best fit the phenotypic profile of T. tyrosinosolvens, whereas those of the isolate recovered from patient 2 best fit that of T. pulmonis.
TABLE 1.
Phenotypic characteristics of the two clinical isolates compared to those of other clinically important Tsukamurella species
| Phenotypic characteristics | Presence or absence in clinical isolate from case:
|
Presence or absence of characteristic in indicated Tsukamurella speciesa:
|
|||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | T. inchonensis | T. paurometabola | T. pulmonis | T. strandjordii | T. tyrosinosolvens | |
| Growth at 42°C | − | − | + | − | − | − | − |
| Hydrolysis of | |||||||
| Tyrosine | + | − | − | − | − | − | + |
| Xanthine | − | − | − | − | − | − | V |
| Assimilation of | |||||||
| Maltose | + | − | + | − | − | V | + |
| Cellobiose | − | − | + | − | − | NA | − |
| d-Melezitose | + | − | + | − | − | V | + |
| d-Sorbitol | + | + | + | − | + | NA | + |
| Glycerol | − | − | V | + | V | V | V |
| 2-Ketogluconate | + | − | V | − | − | V | + |
| Xylitol | − | − | V | − | V | + | V |
| Inositol | + | − | + | − | − | + | + |
| α-Methyl-d-glucoside | − | − | + | − | − | V | V |
| d-Arabinose | + | − | V | − | V | − | V |
| Ribose | − | − | V | − | − | − | V |
| d-Mannose | − | − | + | − | + | + | V |
| Mannitol | + | + | + | − | + | + | + |
| Arbutine | − | − | V | − | − | + | V |
| Salicin | − | − | V | − | − | + | V |
| Inulin | − | − | + | − | − | + | + |
| l-Fucose | + | − | V | − | + | + | + |
| d-Arabitol | + | + | + | − | + | + | + |
16S rRNA gene sequencing.
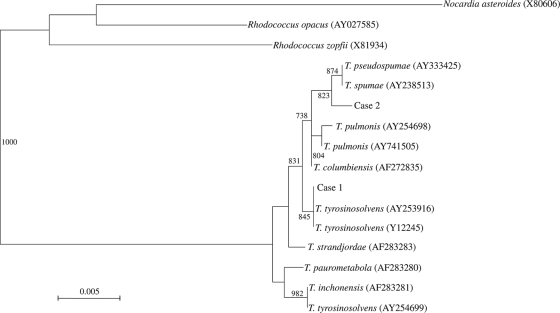
Bacterial DNA extraction, PCR amplification, and DNA sequencing of the 16S rRNA genes of the two isolates of gram-positive bacilli were performed according to our previous publication, using LPW3606 5′-TACTTCGGGATAAGCCTG-3′ and LPW3607 5′-ACGACTTCGTCCCAATCG-3′ (Gibco BRL, Rockville, MD) as the PCR and sequencing primers (24). The sequences of the PCR products were compared with sequences of closely related species in GenBank by multiple sequence alignment, using Clustal_X 1.83 (21). Phylogenetic relationships were determined by using the neighbor-joining method. Sequencing of the 16S rRNA genes of the two isolates showed that there was >99% similarity between the 16S rRNA gene sequences of the two isolates and those of other Tsukamurella species, indicating that both isolates were Tsukamurella species (Fig. 1). There were 0-, 4-, 4-, 6-, and 7-base differences between the 16S rRNA gene sequence of isolate 1 and those of T. tyrosinosolvens (GenBank accession no. FJ643549), T. pulmonis (GenBank accession no. AY741505), T. strandjordae (GenBank accession no. AF283283), T. paurometabola (GenBank accession no. Z37151), and T. inchonensis (GenBank accession no. AF283281), respectively. There were 3-, 3-, 5-, 5-, and 6-base differences between the 16S rRNA gene sequence of isolate 2 and those of T. pseudospumae (GenBank accession no. AY333425), T. spumae (GenBank accession no. AY238513), T. columbiensis (GenBank accession no. AF272835), T. pulmonis (GenBank accession no. AY741505), and T. tyrosinosolvens (GenBank accession no. FJ643549), respectively.
FIG. 1.
Phylogenetic tree showing the relationships of the corneal-scraping isolates from our patients to related species. The tree was inferred from 16S rRNA sequence data by the neighbor-joining method, and bootstrap values were calculated from 1,000 trees. The scale bar indicates the estimated number of substitutions per 200 bases. Names and accession numbers are given as cited in the GenBank database.
Keratitis, or inflammation of the cornea, is caused by both infectious and noninfectious agents. Infective keratitis, an ophthalmologic emergency that requires prompt diagnosis and expedient treatment so as to prevent visual loss, can be caused by bacteria, viruses, fungi, or parasites. Among infectious agents, bacterial pathogens are responsible for the majority of microbial keratitis (1). Most bacterial keratitis is caused by Staphylococcus aureus, Streptococcus pneumoniae, beta-hemolytic streptococci, other gram-positive bacteria such as Bacillus and Propionibacterium species, and other gram-negative bacteria such as P. aeruginosa. In this study, with the help of both phenotypic tests and 16S rRNA gene sequencing, we defined the first two cases of acute keratitis associated with Tsukamurella species.
The symptoms of Tsukamurella keratitis are indistinguishable from those of keratitis caused by other bacteria. Both patients presented with severe eye pain, typical of bacterial keratitis. This is in contrast to three patients from a previous report with Tsukamurella conjunctivitis, which did not cause eye pain (24). Notably, in contrast to keratitis caused by other, more virulent bacteria such as S. aureus and P. aeruginosa, in which there is dense suppurative stromal inflammation and necrosis at the site of bacterial invasion, keratitis in both of our patients was presented as whitish infiltrates in the peripheral parts of the cornea near the limbus, resembling that of marginal keratitis. We speculate that this may be a result of the relatively less virulent nature of the invading Tsukamurella species, so that there was minimal necrosis at the site of initial bacterial invasion. Instead, the inflammatory response began near the limbal vessels from which neutrophils migrate into the cornea, leading to a change in transparency. To ascertain the importance of Tsukamurella species as a cause of keratitis as well as whether the inflammation of most cases of Tsukamurella keratitis appear near the limbus, diphtheroidal gram-positive rods recovered from corneal scrapings should be subjected to detailed phenotypic characterization and 16S rRNA gene sequencing. In fact, cases of Tsukamurella keratitis and conjunctivitis have probably been overlooked in the past because the diphtheroidal gram-positive rods recovered from corneal scrapings and eye swabs were either regarded as contaminants or misidentified as other bacteria, such as atypical mycobacteria (20).
As in most other cases of bacterial keratitis, microtrauma to the epithelial surface of the cornea in both patients in this study predisposed them to Tsukamurella keratitis. In normal individuals, the corneal epithelium and Bowman's membrane underneath prevent penetration of most infectious agents into the corneal stroma unless this natural barrier is breached by trauma. In the first patient of the present study, trichiasis caused constant rubbing of the corneal surface, resulting in an epithelial defect. In fact, she had suffered from corneal abrasion with no infection in the other eye 3 months prior to the present episode of keratitis. For the second patient, contact lens wear had led to microtrauma and an epithelial defect of the corneal surface, which was further aggravated by long hours of use, during which the contact lens can dry out and rub on the cornea (2). We speculate that the Tsukamurella species in the two patients first colonized their corneas. Microtrauma due to trichiasis and contact lens wear, respectively, resulted in epithelial defects which facilitated invasion by the colonized environmental Tsukamurella species, giving rise to keratitis.
Ophthalmologic infections due to Tsukamurella species are associated with T. tyrosinosolvens and T. pulmonis. Among the 10 known species of Tsukamurella, T. inchonensis, T. paurometabola, T. pulmonis, T. strandjordii, and T. tyrosinosolvens have been reported to cause infections in humans (3-11, 13-20, 22-27). Including the present two cases, five ophthalmologic infections due to Tsukamurella species have been described (24). All of these five cases were associated with T. tyrosinosolvens or T. pulmonis (Table 2) (24). On the other hand, of those isolated from sputum, blood, or other clinical specimens, only 16 (53%) of 30 cases with Tsukamurella identified to the species level were due to T. pulmonis or T. tyrosinosolvens (P < 0.05 by Fisher's exact test) (Table 2). We speculate that the genomes of T. tyrosinosolvens and T. pulmonis or “ophthalmologic strains” of T. tyrosinosolvens and T. pulmonis may encode adhesins for binding to unique receptors on the conjunctival and corneal cells or, alternatively, that they may be particularly resistant to antibacterial substances in tears, such as lysozymes, lipocalin, and lactoferrin, leading to the unique susceptibility of the eye to these two species or to “ophthalmologic strains” of these two species.
TABLE 2.
Tsukamurella species isolated from different clinical specimens and reported in the literature
| Sources of specimens | Source or reference(s) | Tsukamurella species (no. of isolates) |
|---|---|---|
| Ophthalmologic specimens | ||
| Eye swab | 24 | T. tyrosinosolvens (2); T. pulmonis (1) |
| Corneal scraping | Present study | T. tyrosinosolvens (1); T. pulmonis (1) |
| Respiratory specimens | ||
| Sputum | 9, 11, 13, 14, 16, 20, 22, 25, 26, 27 | T. paurometabola (3); T. pulmonis (4); T. tyrosinosolvens (3) |
| Necrotic lung tumor | 25 | T. inchonensis (1) |
| Blood specimens | ||
| Blood and catheter tip | 8, 10, 17, 18 | T. paurometabola (3); T. pulmonis (4); T. strandjordii (1); T. tyrosinosolvens (1) |
| Blood | 3, 4, 6, 7, 19, 27 | T. paurometabola (1); T. tyrosinosolvens (4); T. inchonensis (1); T. strandjordii (1) |
| Other specimens | ||
| Cerebrospinal fluid | 15 | T. paurometabola (1) |
| Pus from subcutaneous abscess | 23 | T. paurometabola (1) |
| Skin biopsy | 5 | T. paurometabola (1) |
Nucleotide sequence accession numbers.
The 16S rRNA gene sequences of the two isolates of gram-positive bacilli recovered from the three patients have been lodged within the GenBank sequence database under accession numbers FJ755301 and FJ755300.
Acknowledgments
This work was partly supported by the University Development Fund and the Committee for Research and Conference grant, the University of Hong Kong.
Footnotes
Published ahead of print on 15 April 2009.
REFERENCES
- 1.Asbell, P., and S. Stenson. 1982. Ulcerative keratitis. Survey of 30 years' laboratory experience. Arch. Ophthalmol. 10077-80. [DOI] [PubMed] [Google Scholar]
- 2.Bourcier, T., F. Thomas, V. Borderie, C. Chaumeil, and L. Laroche. 2003. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br. J. Ophthalmol. 87834-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chong, Y., K. Lee, C. Y. Chon, M. J. Kim, O. H. Kwon, and H. J. Lee. 1997. Tsukamurella inchonensis bacteremia in a patient who ingested hydrochloric acid. Clin. Infect. Dis. 241267-1268. [DOI] [PubMed] [Google Scholar]
- 4.Elshibly, S., J. Doherty, J. Xu, R. B. McClurg, P. J. Rooney, B. C. Millar, H. Shah, T. C. Morris, H. D. Alexander, and J. E. Moore. 2005. Central line-related bacteraemia due to Tsukamurella tyrosinosolvens in a haematology patient. Ulster Med. J. 7443-46. [PMC free article] [PubMed] [Google Scholar]
- 5.Granel, F., A. Lozniewski, A. Barbaud, C. Lion, M. Dailloux, M. Weber, and J. L. Schmutz. 1996. Cutaneous infection caused by Tsukamurella paurometabolum. Clin. Infect. Dis. 23839-840. [DOI] [PubMed] [Google Scholar]
- 6.Jones, R. S., T. Fekete, A. L. Truant, and V. Satishchandran. 1994. Persistent bacteremia due to Tsukamurella paurometabolum in a patient undergoing hemodialysis: case report and review. Clin. Infect. Dis. 18830-832. [DOI] [PubMed] [Google Scholar]
- 7.Kattar, M. M., B. T. Cookson, L. D. Carlson, S. K. Stiglich, M. A. Schwartz, T. T. Nguyen, R. Daza, C. K. Wallis, S. L. Yarfitz, and M. B. Coyle. 2001. Tsukamurella strandjordae sp. nov., a proposed new species causing sepsis. J. Clin. Microbiol. 391467-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai, K. K. 1993. A cancer patient with central venous catheter-related sepsis caused by Tsukamurella paurometabolum (Gordona aurantiaca). Clin. Infect. Dis. 17285-287. [DOI] [PubMed] [Google Scholar]
- 9.Maalouf, R., S. B. Mierau, T. A. Moore, and A. Kaul. 2009. First case report of community-acquired pneumonia due to Tsukamurella pulmonis. Ann. Intern. Med. 150147-148. [DOI] [PubMed] [Google Scholar]
- 10.Maertens, J., P. Wattiau, J. Verhaegen, M. Boogaerts, L. Verbist, and G. Wauters. 1998. Catheter-related bacteremia due to Tsukamurella pulmonis. Clin. Microbiol. Infect. 451-53. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto, T., M. Shiraishi, H. Yoshimura, K. Sogen, T. Harada, C. Yoshimura, R. Aramaki, F. Yamamoto, T. Kuraki, and K. Watanabe. 2006. Tsukamurella tyrosinosolvens cultured from sputum of a patient who received total gastrectomy for gastric cancer. Kekkaku 81487-490. (In Japanese.) [PubMed] [Google Scholar]
- 12.Murray, P. R., E. J. Baron, J. H. Jorgensen, M. R. Louise, and M. A. Pfaller (ed.). 2007. Nocardia, Rhodococcus, Gordonia, Actinomadura, Streptomyces, and other aerobic actinomycetes, p. 515-542. In Manual of clinical microbiology, 9th ed. American Society for Microbiology, Washington, DC.
- 13.Osoagbaka, O. U. 1989. Evidence for the pathogenic role of Rhodococcus species in pulmonary diseases. J. Appl. Bacteriol. 66497-506. [DOI] [PubMed] [Google Scholar]
- 14.Perez, V. A., J. Swigris, and S. J. Ruoss. 2008. Coexistence of primary adenocarcinoma of the lung and Tsukamurella infection: a case report and review of the literature. J. Med. Case Rep. 2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prinz, G., E. Bán, S. Fekete, and Z. Szabó. 1985. Meningitis caused by Gordona aurantiaca (Rhodococcus aurantiacus). J. Clin. Microbiol. 22472-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rey, D., D. De Briel, R. Heller, P. Fraisse, M. Partisani, M. Leiva-Mena, and J. M. Lang. 1995. Tsukamurella and HIV infection. AIDS 91379. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz, M. A., S. R. Tabet, A. C. Collier, C. K. Wallis, L. C. Carlson, T. T. Nguyen, M. M. Kattar, and M. B. Coyle. 2002. Central venous catheter-related bacteremia due to Tsukamurella species in the immunocompromised host: a case series and review of the literature. Clin. Infect. Dis. 35e72-e77. [DOI] [PubMed] [Google Scholar]
- 18.Shapiro, C. L., R. F. Haft, N. M. Gantz, G. V. Doern, J. C. Christenson, R. O'Brien, J. C. Overall, B. A. Brown, and R. J. Wallace, Jr. 1992. Tsukamurella paurometabolum: a novel pathogen causing catheter-related bacteremia in patients with cancer. Clin. Infect. Dis. 14200-203. [DOI] [PubMed] [Google Scholar]
- 19.Sheridan, E. A., S. Warwick, A. Chan, M. Dall'Antonia, M. Koliou, and A. Sefton. 2003. Tsukamurella tyrosinosolvens intravascular catheter infection identified using 16S ribosomal DNA sequencing. Clin. Infect. Dis. 36e69-e70. [DOI] [PubMed] [Google Scholar]
- 20.Stanley, T., L. Crothers, M. McCalmont, J. Xu, B. C. Millar, C. E. Goldsmith, and J. E. Moore. 2006. The potential misidentification of Tsukamurella pulmonis as an atypical Mycobacterium species: a cautionary tale. J. Med. Microbiol. 55475-478. [DOI] [PubMed] [Google Scholar]
- 21.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 254876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsukamura, M., and K. Kawakami. 1982. Lung infection caused by Gordona aurantiaca (Rhodococcus aurantiacus). J. Clin. Microbiol. 16604-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsukamura, M., K. Hikosaka, K. Nishimura, and S. Hara. 1988. Severe progressive subcutaneous abscesses and necrotizing tenosynovitis caused by Rhodococcus aurantiacus. J. Clin. Microbiol. 26201-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woo, P. C., A. H. Ngan, S. K. Lau, and K. Y. Yuen. 2003. Tsukamurella conjunctivitis: a novel clinical syndrome. J. Clin. Microbiol. 413368-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yassin, A. F., F. A. Rainey, H. Brzezinka, J. Burghardt, H. J. Lee, and K. P. Schaal. 1995. Tsukamurella inchonensis sp. nov. Int. J. Syst. Bacteriol. 45522-527. [DOI] [PubMed] [Google Scholar]
- 26.Yassin, A. F., F. A. Rainey, H. Brzezinka, J. Burghardt, M. Rifai, P. Seifert, K. Feldmann, and K. P. Schaal. 1996. Tsukamurella pulmonis sp. nov. Int. J. Syst. Bacteriol. 46429-436. [DOI] [PubMed] [Google Scholar]
- 27.Yassin, A. F., F. A. Rainey, J. Burghardt, H. Brzezinka, S. Schmitt, P. Seifert, O. Zimmermann, H. Mauch, D. Gierth, I. Lux, and K. P. Schaal. 1997. Tsukamurella tyrosinosolvens sp. nov. Int. J. Syst. Bacteriol. 47607-614. [DOI] [PubMed] [Google Scholar]