Abstract
BACKGROUND
Hormonal effects of soy and isoflavones have been investigated in numerous trials with equivocal findings. We aimed to systematically assess the effects of soy and isoflavones on circulating estrogen and other hormones in pre- and post-menopausal women.
METHODS
The Cochrane Library, MEDLINE and EMBASE (plus reviews and experts) were searched to December 2007. Inclusion of randomized or residential crossover trials of soy or isoflavones for 4 or more weeks on estrogens, SHBG, FSH, LH, progesterone and thyroid hormones in women was assessed independently in duplicate. Six percent of papers assessed were included. Data concerning participants, interventions, outcomes, potential effect modifiers and trial quality characteristics were extracted independently in duplicate.
RESULTS
Forty-seven studies (11 of pre-, 35 of post- and 1 of perimenopausal women) were included. In premenopausal women, meta-analysis suggested that soy or isoflavone consumption did not affect primary outcomes estradiol, estrone or SHBG concentrations, but significantly reduced secondary outcomes FSH and LH [by ∼20% using standardized mean difference (SMD), P = 0.01 and 0.05, respectively]. Menstrual cycle length was increased by 1.05 days (95% CI 0.13, 1.97, 10 studies). In post-menopausal women, there were no statistically significant effects on estradiol, estrone, SHBG, FSH or LH, although there was a small statistically non-significant increase in total estradiol with soy or isoflavones (∼14%, SMD, P = 0.07, 21 studies).
CONCLUSIONS
Isoflavone-rich soy products decrease FSH and LH in premenopausal women and may increase estradiol in post-menopausal women. The clinical implications of these modest hormonal changes remain to be determined.
Keywords: soy foods, isoflavones, estradiol, sex hormone-binding globulin, gonadotrophins
Introduction
Although interest in the relative importance of isoflavones to human health has increased over the last 15 years, their effects on health are not clearly established. Isoflavones are diphenolic compounds with a range of characterized biological effects from in vitro studies. To date much of the interest in their biologic activity relates to estrogen receptor-mediated mechanisms, given their structural similarity to estrogens (Axelson et al., 1984; Kuiper et al., 1998; Gallo et al., 2005; Hwang et al., 2006; Messina, 2007), but numerous other biological effects independent of estrogen receptors have also been determined [e.g. antioxidant capacity, antiproliferative and anti-inflammatory effects (Kuiper et al., 1998; Setchell and Cassidy, 1999; Gallo et al., 2005; Hwang et al., 2006; Messina, 2007)].
Following modest soy consumption, typical for East Asians, circulating isoflavone concentrations reach the low micromolar level, 100–1000 times that of endogenous estrogen levels, although they circulate predominantly in the less biologically active conjugated form (Axelson et al., 1984; Adlercreutz and Mazur, 1997). These compounds may affect estrogen action by directly binding to estrogen receptors, preferentially estrogen receptor β, thus potentially directly affecting transcription of estrogen-regulated gene products (Kuiper et al., 1997; Pike et al., 1999; An et al., 2001; Lacey et al., 2005; Hwang et al., 2006), acting as estrogen agonists in some contexts and estrogen antagonists in others, much like the selective estrogen receptor modulators tamoxifen or raloxifene. Isoflavones may also influence estrogen action by virtue of effects on enzymes involved in steroid metabolism, including aromatase (Rice et al., 2006), 17β-hydroxysteroid dehydrogenases, steroid sulfatases and sulfotransferases (Lacey et al., 2005), potentially resulting in alterations of circulating estrogens.
Data from human clinical trials evaluating possible beneficial effects of isoflavone-rich products on a variety of health outcomes have been mixed—e.g. some studies suggest that isoflavones inhibit bone loss or alleviate hot flushes (Howes et al., 2006; Williamson-Hughes et al., 2006; Marini et al., 2007; Ma et al., 2008), whereas others have observed no effect (Balk et al., 2005; Nelson et al., 2006; Sacks et al., 2006). Although evidence on health effects in humans is debatable, endocrine modulation in several animal species has been reported following exposure to high levels of isoflavones from soy and red clover (Bennetts et al., 1946; Setchell et al., 1987; Adams, 1995). These hormonal effects in animals have raised questions about the safety of soyfoods (the main source of isoflavones in the human diet), despite a long history of soyfood consumption by many East Asian populations (Messina et al., 2006b).
There are a number of possible explanations for the variability in results among soy studies. A wide variety of intervention products with markedly varying isoflavone content have been used, including traditional soyfoods, isolated soy protein (ISP), soy extracts and isolated isoflavones, each with a variety of controls (Erdman et al., 2004). Other variables include amounts of protein in products, menopausal status of the participants, stage of menstrual cycle in premenopausal women and degree to which dietary intake is controlled.
A systematic evaluation of the literature, ensuring inclusion of the entire set of relevant studies, with greater statistical power to examine the effects of isoflavone-containing soy products on hormonal status, provides improved potential to examine the hormonal effects of soy isoflavones in women. We therefore conducted such a systematic review, and meta-analysis, of the literature to examine the effects of isoflavone-containing soy products on circulating levels of estrogens and other hormones in pre- and post-menopausal women.
Methods
Included studies were required to: be randomized trials or carefully controlled intervention studies (the former had to state that they were randomized or explicitly describe a true randomization method, the latter had to be residential crossover studies that provided and monitored all food and drink intake); be parallel or crossover in design; have an intervention duration ≥4 weeks; be in women ≥16 years old (pre- or post-menopausal, not critically ill, pregnant or breastfeeding); increase intake of soy, soy products or purified soy isoflavones compared with usual diet or usual diet with placebo soy/isoflavone; be unifactorial (so that effects of soy or isoflavones could be separated from those of other interventions); and assess at least one primary or secondary review outcome. Primary outcomes were circulating estradiol, estrone and SHBG in pre- and post-menopausal women. Secondary outcomes were FSH, LH, progesterone, circulating estrone sulfate, circulating free estradiol, thyroid hormones (T4, T3 or TSH), urinary estrogens and estrogenic metabolites, menstrual cycle length, luteal and follicular phase lengths, and IGF-1.
Structured electronic searches were carried out on The Cochrane Library, MEDLINE, EMBASE and the Meta-register of controlled trials (http://www.controlled-trials.com/mrct/), from inception to December 2007, in the format: [soy or isoflavones] AND [hormones] AND [randomized controlled trials]. We also checked the reference lists of large non-systematic reviews of trials of soy and isoflavone to ensure studies assessing hormones as secondary outcomes were not missed. Experts were contacted to obtain further (and unpublished) trials. Studies were not limited by publication status (whether fully published, published in abstract form, or unpublished) or language of publication.
Resulting titles and abstracts were assessed independently in duplicate by two reviewers and full text articles collected. Inclusion was assessed independently in duplicate by two reviewers, and disagreements resolved by discussion. Included papers were grouped into studies and data (on participants, interventions, outcomes at the latest time point to 53 weeks, trial quality characteristics and potential effect modifiers) were extracted independently by two reviewers onto a data extraction form refined by the entire review team.
Trial quality characteristics assessed included: masking (separately) of participants and outcome assessors (coded as ‘yes’ where there was a clear and realistic attempt to mask, ‘no’ where not, or ‘unclear’—success of masking was rarely checked in included studies); industry funding or involvement (any funding, including full funding of the study, co-authorship of a scientist or statistician working for industry or provision of materials to be used during the intervention and/or control, and coded as ‘yes, industry funding’, ‘none reported’ or ‘unclear’); duration (coded as ‘done’ for all post-menopausal studies of at least 4 weeks in duration, and premenopausal studies of at least 3 cycles duration or ‘not done’ for shorter premenopausal studies); assessment and reporting of compliance (‘done’ when compliance was both assessed and reported, ‘partly done’ when it was assessed but not reported or reported without any indication of the method used, and ‘not done’ when neither was addressed adequately); isoflavone content (reported as ‘done’ when total isoflavone, genistein and daidzein contents reported in both intervention and control, aglycone or glycated form reported, ‘partially done’ when at some of the above completed, ‘not done’ when not); isoflavones analyzed (‘done’ when the intervention dose was checked and reported, or ‘unclear’ if not carried out or not reported); and dropouts [reported as ‘done’ when the numbers of participants who were randomized, completed and analyzed in each arm were all clear, and reasons for dropouts were given (by intervention arm), ‘partially done’ when some of the above, ‘not done’ when none of these data were presented]. Trials were considered to be at low risk of bias if participant and outcome assessor blinding were all coded ‘yes’, industry funding was not reported, duration was done and dropouts ‘done’. All other trials were considered at moderate or high risk of bias.
For premenopausal studies, it was not feasible to choose any single phase of the menstrual cycle for data extraction (i.e. data were measured at different points during the cycle and selection of day of sampling within phase varied). Thus, we chose the point in each study with the highest control group baseline measurement for each outcome. This was based on the premise that it is the level and timing of the peak of hormone concentration that is important in determining health effects rather than the baseline levels present at other times of the cycle. This approach, while not ideal, was based on the premise that this was the point at which any changes due to soy or isoflavones might be most easily detected. To check that this assumption did not result in missing effects based on the use of non-peak data, we re-ran the analyses using data from the luteal phase only for estrogens, FSH and LH, and the follicular phase for progesterone.
Isoflavone dose was calculated in aglycone equivalents (glycoside levels were multiplied by 0.6). For primary and secondary outcomes, the number of participants assessed, means and standard deviations of change from baseline (where available, or data at the end of the intervention and control periods where change data were not available) in hormonal concentration in each treatment arm were extracted. For crossover studies, we aimed to use within-participant differences with the variance of these differences (Elbourne et al., 2002); however, the information on variance for the within-participant differences were not provided in our included studies, and without at least 2 studies providing such data, it was not possible to impute appropriate variance estimates with any confidence (Follman et al., 1992). For this reason, and because exact P-values or t-statistics were only available for occasional outcomes in a few of the crossover studies (generally being reported as ‘significant’ or not), mean and variance for the participants while on each treatment were used instead, treating results from the intervention period as if they came from one group of patients and results from the control period as if they came from a second group of patients. This is not ideal as the two groups are not independent (as required for the statistical tests) and will tend to provide a conservative estimate of any association as it ignores the within-patient correlations that give crossover trials their statistical strength (Elbourne et al., 2002). We ran a sensitivity analysis omitting crossover data except where an exact t-test or P-value for the relationship between the two periods was available. Heterogeneity was assessed using I2 (Higgins et al., 2003). Data for pre- and post-menopausal women were analyzed separately.
Owing to varied mean baseline hormone concentrations of many hormones (e.g. for post-menopausal women, where menstrual phase was not relevant, mean total estradiol ranged from <20 to almost 200 pmol/l at baseline), we decided that our primary analyses would combine studies using standardized mean differences (SMD) in random effects meta-analysis, where at least two studies were combined. This was because the studies were effectively using different measurement scales to assess the same effect size. The SMD ‘expresses the size of the intervention effect in each study relative to the variability observed in that study’ (Anon., 2008; Deeks et al., 2008). Interpretation of the effect size is problematic as the units are units of standard deviation rather than the more intuitive pmol/l or equivalent ‘real’ units. For cycle lengths, studies were combined using mean differences (MD) in random effects meta-analysis. As a check on our results (sensitivity analysis), we also ran the meta-analyses using MD in place of SMD, and assessed robustness of results to trial quality (trials assessed as at moderate or high risk of bias were removed).
Subgroup analyses explored the effects of the following factors on the primary outcomes: intake of soy protein (<10, 10–24, 25–49, 50+ g/day of soy protein); isoflavone dose (<25 mg/day, 25 to <50 mg/day, 50 to <75 mg/day, 75 to <100 mg/day, 100+ mg/day); intervention intensity (dietary advice, supplementation or food provided); equol producers versus non-producers; and isoflavone source. We assessed for evidence of dissemination bias using funnel plots.
Results
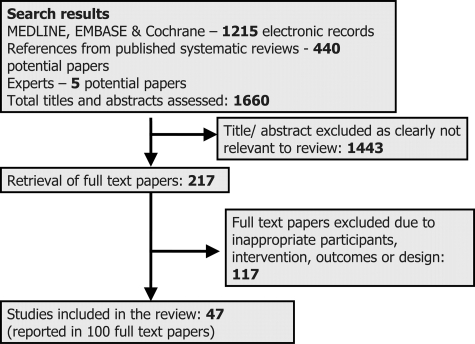
Searches identified 1660 titles and abstracts for assessment (Fig. 1), 217 were retrieved in full text, and 47 studies were included and data extracted. Details of the included studies are provided in the appendix.
Figure 1.
Study flow diagram of the search process and inclusion of studies into the review.
Eleven included studies were of premenopausal women (579 women analyzed), 35 of post-menopausal women (1165 analyzed), and 1 of perimenopausal women (69 analyzed). Six studies, all of post-menopausal women, provided no useable primary or secondary outcome data, despite requests from authors (data were only provided as least squares means with associated reduction in standard errors, did not separate out women using HRT, stated presence or absence of statistical significance but provided no actual data or did not present variances). However, these studies were included in the review to help us understand the quantity of missing data, and allow us to assess the potential of studies not included in the meta-analyses to alter the results of the pooling (Table I). As outcome reporting may vary according to whether statistically significant effects were seen, we did not want to assume that studies with missing data would be similar to those with presented data. Studies ranged in size from 10 to 304 women analyzed (mean 59). Thirty-two included studies were parallel in design, and 15 crossover. Nineteen studies assessed the effect of isoflavone extract versus control, nine isoflavone-containing ISP versus isoflavone-depleted ISP, 13 ISP versus another control, nine whole soy or soy foods versus control (some studies included more than one comparison). Studies ranged from 4 to 104 weeks long: 29 were 4–12 weeks in duration; nine were 13–26 weeks; seven 27–52 weeks; and two >1 year. One was conducted in metabolic ward conditions, the remainder in the community (three of these provided all food for the intervention period). Twenty-five studies were carried out in North America, nine in Europe, five in Asia and four each in Australia and South America.
Table I.
Table of primary and secondary outcomes
| Premenopausal women |
Post-menopausal women |
|||||||
|---|---|---|---|---|---|---|---|---|
| No. of studies/participantsa/missing studiesb | SMD (95% CI) | I2 (%)c | MD (95% CI) | No. of studies/participantsa/missing studiesb | SMD (95% CI) | I2 (%)c | MD (95% CI) | |
| Primary outcomes | ||||||||
| Circulating total E2, pmol/l | 11/250/0 | −0.05 (−0.23 to 0.12) | 0 | −7.99 (−48.20 to 32.22) | 21/580/6 | 0.13 (−0.01 to 0.27) | 29.3 | 2.76 (−0.37 to 5.90) |
| Circulating total E1, pmol/l | 6/207/1 | −0.09 (−0.29 to 0.10) | 0 | −12.21 (−36.60 to 12.17) | 7/152/1 | −0.13 (−0.36 to 0.10) | 0 | −5.33 (−11.56 to 0.90) |
| Circulating SHBG, nmol/l | 10/233/1 | −0.10 (−0.28 to 0.08) | 0 | −2.19 (−6.37 to 1.99) | 17/459/3 | −0.06 (−0.19 to 0.07) | 0 | −0.87 (−3.52 to 1.78) |
| Secondary outcomes | ||||||||
| Circulating FSH, IU/l | 7/73/0 | −0.45 (−0.79 to −0.11) | 0 | −0.52 (−1.15 to 0.11) | 23/601/4 | −0.08 (−0.26 to 0.10) | 59.3 | −1.29 (−4.41 to 1.83) |
| Circulating LH, IU/l | 7/73/0 | −0.34 (−0.68 to −0.01) | 0 | −1.26 (−3.30 to 0.78) | 13/382/2 | 0.01 (−0.21 to 0.24) | 58.1 | 0.27 (−2.42 to 2.95) |
| Circulating progesterone, nmol/l | 9/199/0 | 0.03 (−0.29 to 0.36) | 44.0 | −1.20 (−6.89 to 4.48) | 0/0/0 | — | — | — |
| Circulating E1S, nmol/l | 3/44/0 | 0.09 (−0.34 to 0.52) | 0 | 0.49 (−2.12 to 3.11) | 6/125/2 | −0.03 (−0.29 to 0.22) | 0 | −0.03 (−0.28 to 0.22) |
| Circulating free E2, pmol/L | 3/145/0 | −0.09 (−0.32 to 0.14) | 0 | −0.71 (−2.21 to 0.79) | 3/77/0 | 0.07 (−0.25 to 0.39) | 0 | 0.18 (−0.10 to 0.46) |
| Circulating T3, nmol/l | 1/14/0 | — | −0.03 (−0.11 to 0.05) | 2/41/1 | 0.29 (−0.14 to 0.72) | 0 | 0.11 (−0.03 to 0.26) | |
| Circulating T4, nmol/l | 1/14/0 | — | −1.60 (−6.73 to 3.53) | 3/59/1 | 0.31 (−0.05 to 0.67) | 0 | 3.53 (−0.61 to 7.68) | |
| Circulating TSH, mU/l | 1/14/0 | — | −0.03 (−0.86 to 0.80) | 7/192/1 | −0.01 (−0.21 to 0.19) | 0 | 0.05 (−0.26 to 0.35) | |
| Circulating IGF-1, nmol/l | 3/167/1 | 0.14 (−0.07 to 0.35) | 0 | 0.75 (−0.60 to 2.11) | 3/209/2 | 0.29 (−0.39 to 0.97) | 80.7 | 1.57 (−1.75 to 4.89) |
| Urinary E2, nmol/24 h | 1/11/3 | — | −1.81 (−2.89 to −0.73) | 1/18/0 | — | 0.05 (−0.64 to 0.74) | ||
| Urinary E1, nmol/24 h | 1/14/3 | — | −2.34 (−4.80 to 0.12) | 1/18/0 | — | −0.57 (−1.68 to 0.54) | ||
| Menstrual cycle length, days | 10/148/1 | 0 | 1.05 (0.13 to 1.97) | |||||
| Luteal phase length, days | 3/44/0 | 0 | 0.54 (−0.32 to 1.40) | |||||
| Follicular phase, days | 7/92/0 | 7.6 | 0.81 (−0.12 to 1.74) | |||||
SMD, standardized mean difference; MD, mean difference; I2, I2 test of heterogeneity; 95% CI, 95% confidence interval; E1, estrone; E2, estradiol; E1S, estrone sulfate.
aNumber of participants in the control arms of all included studies combined.
bNumber of studies that have data available on this outcome, but where those data are not useable in meta-analysis.
cI2 relates to the SMD except where no SMD data appear (in which case, it relates to MD).
Study quality varied, as shown in Supplemental data Table S1. All but one of the included studies was randomized, and the non-randomized study was a closely supervised metabolic crossover study (Cassidy et al., 1995). Thirty-five studies masked participants (4 did not and in 8 studies this was unclear) and 31 studies masked outcome assessors (3 did not, 13 unclear). Twenty studies declared a funding source with a commercial interest in the study results; 25 did not report industry funding; and 2 were unclear. Duration of intervention was adequate in 42 studies, not in 5. Compliance was assessed and reported in nine studies, partly done in 24, and not done in 14. Isoflavone dose was well reported in 18 studies, partly in 27, and not in 2. Isoflavones were analyzed in 17 studies, not in one study, unclear in 29. Dropouts were fully reported in 25 studies, partly reported in 20, not in 2. Ten studies were judged at low risk of bias.
Primary outcomes
Summary effect data are presented in Table I, with sensitivity analyses omitting crossover study data unless t-test or exact P-values were available in Table II. Summary forest plots (for outcomes with at least four studies contributing data) are shown in Figs 2 (for premenopausal women) and 3 (post-menopausal women).
Table II.
Sensitivity analysis of primary and secondary outcomes, removing crossover data except where exact t-test statistic given
| Premenopausal women |
Post-menopausal women |
|||
|---|---|---|---|---|
| No. of studies/participantsa | SMD (95% CI) | No. of studies/participantsa | SMD (95% CI) | |
| Primary outcomes | ||||
| Circulating total E2, pmol/l | 4/347 | −0.08 (−0.30 to 0.13) | 17/940 | 0.15 (−0.02 to 0.32) |
| Circulating total E1, pmol/l | 4/347 | −0.09 (−0.30 to 0.12) | 4/150 | −0.14 (−0.59 to 0.30) |
| Circulating SHBG, nmol/l | 4/344 | −0.13 (−0.34 to 0.09) | 13/750 | −0.07 (−0.22 to 0.09) |
| Secondary outcomes | ||||
| Circulating FSH, IU/l | 2/40 | −0.87 (−1.72 to −0.02) | 15/834 | −0.05 (−0.32 to 0.22) |
| Circulating LH, IU/l | 2/41 | −0.46 (−1.16 to 0.25) | 10/605 | 0.05 (−0.25 to 0.34) |
| Circulating progesterone, nmol/l | 2/219 | 0.14 (−0.56 to 0.83) | 0/0 | — |
| Circulating E1S, nmol/l | 1/29 | MD: 1.88 (−2.40 to 6.16) | 2/77 | 0.12 (−0.33 to 0.57) |
| Circulating free E2, pmol/l | 3/284 | −0.09 (−0.32 to 0.14) | 2/85 | 0.15 (−0.30 to 0.60) |
| Circulating T3, nmol/l | 0/0 | 2/85 | 0.29 (−0.14 to 0.72) | |
| Circulating T4, nmol/l | 0/0 | 2/85 | 0.29 (−0.15 to 0.73) | |
| Circulating TSH, mU/l | 0/0 | 5/289 | −0.03 (−0.26 to 0.20) | |
| Circulating IGF-1, nmol/l | 3/338 | +0.14 (−0.07 to 0.35) | 2/387 | 0.59 (0.38 to 0.79) |
| Menstrual cycle length, days | 3/155 | MD: 0.89 (−0.61 to 2.39) | ||
SMD, standardized mean difference; MD, mean difference; 95% CI, 95% confidence interval; E1, estrone; E2, estradiol; E1S, estrone sulfate.
aNumber of participants in the intervention and control arms of all included studies combined.
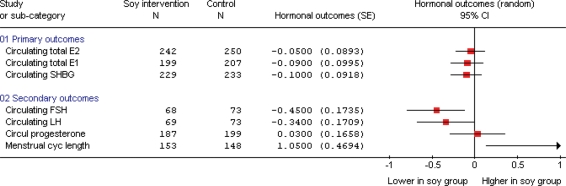
Figure 2.
Effects of soy and isoflavones on circulating hormones and menstrual cycle length in premenopausal women (including all outcomes where at least four studies contributed to the data). SMD analysis, all in units of standard deviation, except for menstrual cycle length, which is a MD analysis, where the units are days.
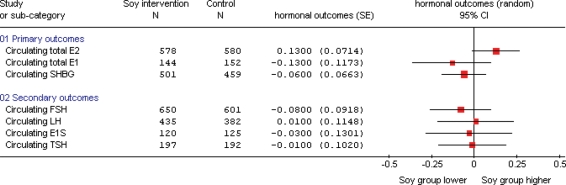
Figure 3.
Effects of soy and isoflavones on circulating hormones in post-menopausal women (presenting all outcomes where at least four studies contribute to the data). SMD analysis, all in units of standard deviation.
Premenopausal women
Soy and isoflavone consumption had no effect on circulating total estradiol, estrone or SHBG concentrations in premenopausal women (on the basis of 6–11 studies per comparison, each comparison had over 200 women in combined control arms, combining with either SMD or MD, no suggestion of heterogeneity). One further study assessed the effect of soy isoflavones on circulating total estrone and SHBG (data not useable in our meta-analysis), but addition of these results would be unlikely to significantly alter the outcome. Using data only from the luteal phase (rather than the point with the highest baseline measure) for total estradiol and estrone resulted in no suggestions of an effect (circulating estradiol SMD 0.03, 95% CI −0.17 to 0.23, I2 0%; circulating estrone SMD 0.00, 95% CI −0.23 to 0.23, I2 0%). Removing data for crossover studies except where exact t-test or P-values were available resulted in similar results to the main analysis, except that confidence intervals were generally widened slightly due to the loss of some power.
Post-menopausal women
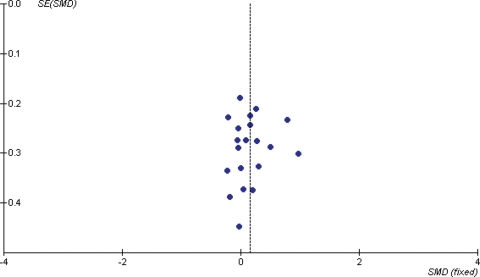
In post-menopausal women, there was a small increase in circulating total estradiol concentrations following soy isoflavone consumption, but this was not statistically significant (on the basis of 21 studies, 580 women in combined control groups, SMD 0.13, 95% CI −0.01 to 0.27, or an increase of 14%, 95% CI −1% to +29%, P = 0.07, I2 29.3%), see Supplementary Fig. 1. Sensitivity analysis using MD was not statistically significant (MD 2.76 pmol/l, 95% CI −0.37 to 5.90), nor was the sensitivity analysis removing studies not assessed as at low risk of bias (SMD 0.17, 95% CI −0.07 to 0.41, eight studies, 231 in control groups, I2 37.7%), or that removing the underpowered crossover studies (SMD 0.15, 95% CI −0.02 to 0.32, P = 0.09, I2 39.2%). A funnel plot (Fig. 4) suggested that studies finding more extreme increases and decreases of estradiol following soy isoflavone intervention may be missing from the review. A further six studies (including 267 control participants) had analyzed the effect of soy isoflavones on total circulating estradiol in post-menopausal women, but not presented the data in a useable way—addition of the results of these studies could alter both the effect size and the statistical significance of the results of this meta-analysis.
Figure 4.
Funnel plot of results from included published studies on the effects of soy protein and isoflavones on circulating total estradiol (E2, pmol/l) in post-menopausal women.
Soy isoflavones had no effect on circulating total estrone (7 studies, 152 in control) or SHBG concentrations (17 studies, 459 women in control groups), combining by either SMD or MD, with no suggestion of important heterogeneity. There were one and three studies, respectively, that clearly assessed estrone and SHBG in post-menopausal women, but did not present data in a useable format—addition of these studies would be unlikely to appreciably alter the results.
Subgrouping
Subgrouping by isoflavone source indicated that consumption of isoflavone extracts (rather than soy foods or ISP) was associated with a significant increase in circulating estradiol among post-menopausal women (Supplementary Fig. 1). The SMD of isoflavone extracts was 0.21 (95% CI 0.03 to 0.40, 14 studies, 398 in control groups, I2 40.1%). However, given the total number of subgroups analyzed, and the proximity of the confidence interval for this subgroup to zero, this finding may be due to chance alone.
Subgrouping by total isoflavone dose or soy protein intake resulted in no suggestion of an effect moderated by either. It was not possible to subgroup by equol-producer status (as not enough studies reported data on equol concentrations) or by intensity of intervention (as all studies provided a supplement or food and none provided advice alone).
Secondary outcomes
Premenopausal women
Soy isoflavones reduced circulating FSH and LH, and increased menstrual cycle length in premenopausal women, as shown in Supplementary Figs 2–4. Effects on FSH and LH were observed in seven studies (73 participants in control groups) and effects were statistically significant using SMD, but not MD. I2 was 0%, suggesting no important heterogeneity between the studies. Removing studies not at low risk of bias resulted in no remaining studies (trials were considered to be at low risk of bias if participant and outcome assessor blinding were all coded ‘yes’, industry funding was not reported, duration was done and dropouts ‘done’, all other trials were considered at moderate or high risk of bias—for further details, see definition below Supplementary Table S1) whereas removing crossover studies without exact t-test or P-values resulted in wider confidence intervals, with the effect on FSH remaining statistically significant and loss of such significance for LH and menstrual cycle length.
Interpretation of SMD is problematic as it uses units of standard deviation, but we can use the largest single study (Maskarinec 2002, with 16 participants in the control group) to provide insight into the magnitude of the effect using conventional units. In that study, the control group FSH was 5.4 IU/l at the study end, so that the effect of soy isoflavones on FSH corresponded to −1.2 IU/l (95% CI −0.3 to −2.2), a decrease of ∼22%. Control group LH concentrations at the study end were 4.5 IU/l, giving an effect size of −1.1 IU/l (95% CI −0.03 to −2.2), a decrease of ∼24%. All studies that determined the effects of soy isoflavone intervention on FSH or LH in premenopausal women were useable in meta-analysis.
Combined analysis of 10 studies (148 women in control groups) suggested an increase in menstrual cycle length of 1.1 days with soy isoflavones compared with control. Sensitivity analysis, removing studies not at low risk of bias, removed statistical significance (WMD 1.21 days, 95% CI −0.98 to 3.41, two studies, 62 women in control groups, I2 28.3%).
Urinary estradiol concentrations were reduced in women taking soy isoflavones, based on only one study of 14 women. Soy isoflavones had no statistically significant effects on progesterone, circulating free estradiol or IGF-1 concentrations (where there were at least three studies and at least 50 women in combined control groups). There were insufficient data to comment on the effects on circulating estrone sulfate, T4, T3, TSH, urinary estrone or urinary estradiol.
Using data only from the luteal phase (rather than the point with the highest baseline measure) for circulating FSH and LH, and only from the follicular phase for progesterone, resulted in no suggestions of an effect (circulating FSH SMD −0.07, 95% CI −0.42 to 0.27, I2 0%; circulating LH SMD 0.05, 95% CI −0.27 to 0.36, I2 0%; circulating progesterone SMD −0.02, 95% CI −0.30 to 0.26, I2 17%).
Post-menopausal women
Soy isoflavone intake had no effect on FSH, LH, circulating estrone sulfate, circulating free estradiol, TSH, T4 or IGF-1 concentrations (where there were at least three studies and at least 50 women in combined control groups). There were insufficient data to comment on the effects on progesterone, T3, urinary estrone or urinary estradiol.
Perimenopausal women
Two studies included some perimenopausal women, but only Alekel 2000 provided useable data for meta-analysis. Effects on circulating estrone, circulating estradiol, FSH and IGF-1 were all non-significant (24 women in the control group).
Side effects
Gastrointestinal side effects, but not dropouts due to adverse effects or any recorded side effect, were statistically significantly more likely to occur in participants taking any source of soy and/or isoflavones than controls (pre- and post-menopausal women combined, gastrointestinal side effects RR 1.8, 95% CI 1.3 to 2.6, eight studies, 507 in control groups, I2 0%; any side effect RR 1.9, 95% CI 0.5 to 8.0, two studies, 131 in control groups, I2 79%; dropouts due to adverse events RR 1.6, 95% CI 0.7 to 3.7, four studies, 357 in control groups, I2 25%).
Discussion
This systematic review of 47 studies assessed the effects of soy isoflavones on hormone concentrations in pre- and post-menopausal women. In premenopausal women, consumption of soy isoflavones had no effect on circulating total estradiol, estrone or SHBG. There were significant reductions in FSH (by ∼22%, P = 0.01) and LH concentrations (by ∼24%, P = 0.05), and an increase in menstrual cycle length of 1.05 days (95% CI 0.13 to 1.97, I2 0%, 10 studies with 148 women in control groups) as shown in Table I and Fig. 3. However, these effects should be considered tentative because in sensitivity analysis, when only studies at low risk of bias were retained, the results were no longer statistically significant. No statistically significant effects were observed on free estradiol, progesterone or IGF-1 concentrations.
In post-menopausal women, there were no statistically significant effects of soy or isoflavones on circulating total estradiol, estrone or SHBG, although there was a small non-significant increase in total circulating estradiol following soy (∼14%, P = 0.07, 21 studies, 580 women analyzed in control groups). Soy had no effect on FSH, LH, free estradiol or estrone sulfate, T4, IGF-1 or TSH.
These data are consistent with at most weak effects of soy isoflavones on the hypothalamic–pituitary–gonadal axis in women. They could occur via the effects of isoflavones on endogenous estrogen synthesis, through alterations of enzymes involved in steroid metabolism, including aromatase (Rice et al., 2006), 17β-hydroxysteroid dehydrogenases, steroid sulfatases and sulfotransferases (Lacey et al., 2005). Alternatively, isoflavones may exert estrogenic or anti-estrogenic effects by binding to estrogen receptors, directly affecting transcription of estrogen-regulated gene products (Kuiper et al., 1997; Kuiper et al., 1998; Pike et al., 1999; Rosselli et al., 2000; An et al., 2001). Although isoflavones have a weaker binding affinity for estrogen receptors than endogenous estrogens, circulating levels of isoflavones following consumption of soy will exceed endogenous estrogen levels by several orders of magnitude (Axelson et al., 1984; Cassidy et al., 1994; Adlercreutz and Mazur, 1997). Our data, however, suggest no changes in estrogen status in premenopausal women and borderline effects in post-menopausal women. This lack of effect contrasts with notable and often-cited animal data; for example, infertility in Western Australian sheep grazing on isoflavone-rich clover (Bennetts et al., 1946; Adams, 1995) and captive North American cheetahs consuming diets containing soy protein (Setchell et al., 1987). However, in both cases, circulating levels of isoflavones were much higher than could realistically be achieved in humans. In Australian sheep, this was because of the high isoflavone exposure and in the cheetah, due to their inability to glucuronidate isoflavones in vivo.
Whether the observed but tentative premenopausal changes in FSH and LH reflect an estrogenic or anti-estrogenic effect is not clear. These hormones were assessed in different studies at different points in the menstrual cycle, including, for example, during the midcycle gonadotrophin surge, when a decrease in LH is best construed as an anti-estrogenic effect, while during the luteal phase a decrease in LH may be an estrogenic effect. On the other hand, the increase in menstrual cycle length suggests an anti-estrogenic effect, with longer cycles linked to reduced breast cancer risk (Setchell et al., 1984; Kelsey et al., 1993; Cassidy et al., 1994; Duncan et al., 1999a; Messina et al., 2006a), and a growing body of evidence that increased lifetime soy exposure lowers breast cancer risk (Wu et al., 2008).
In post-menopausal women, the small statistically non-significant increase in circulating estradiol concentrations is potentially of concern, as a recent meta-analysis of nine prospective studies showed that increased levels of circulating estradiol were associated with an increased risk of breast cancer in post-menopausal women (Endogenous Hormones and Breast Cancer Collaborative Group, 2002). Being in the top quintile of total estradiol, compared with the bottom quintile, doubled breast cancer risk (RR 2.00, 95% CI 1.47 to 2.71). However it is difficult to assess the absolute effect of the small statistically non-significant increase in circulating estradiol associated with increased intake of soyfoods and supplements observed in our systematic review. In this regard, the fact that neither SHBG nor LH and FSH concentrations were affected argues against a physiologically important estrogenic effect. Furthermore, the available clinical and epidemiological data do not support the idea that soy isoflavone exposure increases breast cancer risk (Messina et al., 2006a; Wu et al., 2008) or menopausal symptoms (Nelson et al., 2006; Lethaby et al., 2007), although there is conflicting and limited evidence that isoflavones from red clover reduce hot flush frequency in menopausal women (Nelson et al., 2006; Coon et al., 2007).
There are many limitations of the data set used in this systematic review and meta-analysis. The diversity of baseline and control group values between studies for most hormones [also seen in prospective studies (Endogenous Hormones and Breast Cancer Collaborative Group, 2002)] was managed by assuming that different studies were effectively using different scales to measure these hormones (e.g. variation in the methodology employed to assay a particular hormone may have generated consistent among-study variation in hormone concentrations), and by carrying out meta-analysis using SMD. Many data appeared to be skewed, so group means may be dependent on a few extreme values and thus unreliable. Some authors appropriately dealt with this by presenting their data as medians or geometric means (only the latter being amenable to meta-analysis). Other studies presented least squares means following model adjustment, but these were not combinable in meta-analysis due to associated smaller standard errors (so would have been weighted incorrectly). Many parallel studies were small, with baseline hormone levels that differed a great deal between the intervention and the control groups (and where changes over time were smaller than the initial difference between the groups, so that outcome data represented baseline levels more strongly than changes engendered by time or the intervention). On the other hand, the crossover data were underweighted in the analyses due to lack of information on the within-participant differences in the studies. We checked that these studies were not providing misleading data by running a sensitivity analysis removing crossover studies without exact t-test or P-values, and found that the effects were not greatly altered, but that confidence intervals were widened due to reduced power in the remaining studies. Another issue was how to combine data measured at different points during the menstrual cycle. Our decision to take the point at which control data were greatest was not ideal, but appeared the only realistic approach. Finally, a large number of studies assessed the effects of soy isoflavones on at least one of the selected outcomes, but did not report the data in a way that was useable in meta-analysis. As far as possible, we collected details of these missing data to document the extent of the problem.
To our knowledge, this is the first systematic review and meta-analysis to compare the endocrine effects of different soy products on hormonal status in women at different lifecycle stages. It provides weak evidence that soy and isoflavones decrease FSH and LH in premenopausal women, and a suggestion that they may increase estradiol in post-menopausal women. The clinical implications of these relatively modest hormonal changes are unclear and the clinical relevance of these findings for women at different stages of the lifecycle require confirmation in further robust studies.
Author's role
L.H. and A.C. were the principal investigators and the study was initiated by discussion between A.C. and M.J.M. L.H. led the review and is the guarantor of the study. The protocol was drafted by L.H., A.C. and M.J.M., and all authors contributed to, and agreed, the final protocol. J.J.R. and L.H. ran the searches, assessed titles and abstracts for collection, collected relevant full text papers, assessed these for inclusion and merged papers into studies. J.J.R. organized distribution of full text papers to authors for data extraction. All authors contributed to development of the final data extraction form, agreed review methodology and data extracted a share of the included studies. J.J.R., A.C. and L.H. collated the duplicated data extraction forms and adjudicated where differences emerged. J.J.R. and L.H. collated data into tables and ran the analyses in RevMan. L.H. and A.C. prepared the first draft of the paper, and all authors contributed significantly to data interpretation, and preparation of the second draft of the manuscript. All authors agreed the final draft.
Supplementary data
Supplementary data are available at http://humupd.oxfordjournals.org/.
Funding
This work was partially funded by the Soy Nutrition Institute, Inc., St Louis, MO, USA. M.J.M. consults for companies that manufacture and/or sell soyfoods, soy protein and isoflavone supplements and is a Scientific Advisory Board Member of the Soy Nutrition Institute.
Supplementary Material
Acknowledgements
Many thanks to the trialists who took the time to reply, provide additional information, expertise and data for the review: D Lee Alekel (Iowa State University, Alekel 2000); Bahram Arjmandi (Florida State University, Arjmandi 2003, 2005); Francesco Branca (World Health Organization, Copenhagen, Brink 2008); Elisabeth Brink (TNO Quality of Life, Zeist, The Netherlands, Brink 2008); Blakely D. Brown (University of Montana, Brown 2002); Michael Daskalakis (Southern Cross Pathology, Australia, Kotsopoulos 2006); Alison Duncan (University of Guelph, Duncan 1999 pre and post); J.A. Eden (University of New South Wales, Australia, Knight 2001); Christopher Gardner (Stanford University School of Medicine, Gardner 2001); Barry Goldin (Tufts University School of Medicine, Lichtenstein 2002); Jinzhu Liu (Barbara Gross Research Centre, Randwick, Australia, Knight 2001); Gertraud Maskarinec (Cancer Research Center of Hawaii, Maskarinec 2004); Jennifer A. Nettleton (University of Minnesota, Nettleton 2004); Eini Nikander (Helsinki University Central Hospital, Nikander 2004); Giovanni Scambia (Catholic University of the Sacred Heart, Rome, Scambia 2000); Helena Teede (Department of Medicine, Dandenong, Australia, Kotsopoulos 2006); Lillian U. Thompson (University of Toronto, Brooks 2004); Margo Woods (Tufts University School of Medicine, Woods 2000); Michelle Warren (Center for Menopause and Hormone Disorders, New York, Upmalis 2000); Anna Wu (University of Southern California, Wu 2005).
Appendix: Characteristics of included studies
| Study | Participants | Interventions | Outcomes |
|---|---|---|---|
| Alekel 2000 | Participants: peri-men (com) | Design: parallel | DO: 11, unclear from which arms |
| USA (Alekel et al., 2000; Dent et al., 2001; St Germain et al., 2001; Swain et al., 2002; Moeller et al., 2003) | Analyzed: int 24, cont A 21, cont B 24 | Intervention:A: ISP versus milk protein | Duration: 24 weeks |
| Med age: int 50, cont A 49, cont B 51 | B: ISP versus alcohol-washed ISP | ||
| Isoflav, mg/day: int 80, cont A unclear, cont B 4.4 (AU) | |||
| Soy protein difference: 0 g/day | |||
| Arjmandi 2003 | Participants: post-men (com) | Design: parallel | DO: int 16, cont 13 |
| USA (Arjmandi et al., 2003, 2004) | Analyzed: int 20, cont 22 | Intervention: ISP versus milk protein | Duration: 12 weeks |
| Mean age: int 62, cont 62 | Isoflav, mg/day: int 88.4, cont 0 (AU) | ||
| Soy protein difference: 37 g/day | |||
| Arjmandi 2005 | Participants: post-men (com) | Design: parallel | DO: int 13, cont 12 |
| USA (Arjmandi et al., 2005) | Analyzed: int 35, cont 27 | Intervention: ISP versus no soy | Duration: 52 weeks |
| Mean age: int 53, cont 56 | Isoflav, mg/day: int 60, cont unclear (AU?) | ||
| Soy protein difference: 25 g/day | |||
| Aubertin-Leheudre 2007 | Participants: obese post-men (com) | Design: parallel | DO: 14 in each arm |
| Canada (Aubertin-Leheudre et al., 2007) | Analyzed: int 10, cont 10 | Intervention: isoflav ext versus placebo | Duration: 52 weeks |
| Mean age, sd: 58 overall | Isoflav, mg/day: int 70, cont unclear (AU?) | ||
| Soy protein difference: 0 g/day | |||
| Baird 1995 | Participants: post-men (com) | Design: parallel | DO: 6 (unclear from which arms) |
| USA (Baird et al., 1995) | Analyzed: int 66, cont 25 | Intervention: whole soy versus usual diet | Duration: 4 weeks |
| Mean age: unclear | Isoflav, mg/day: int 165, cont unclear (AU?) | ||
| Soy protein difference: unclear | |||
| Baum 1998 | Participants: HC post-men (com) | Design: parallel | DO: 15 (unclear from which arms) |
| USA (Baum et al., 1998; Persky et al., 2002; Potter et al., 1998) | Analyzed: ISP90 21, ISP56 23, cont 22 | Intervention: ISP versus milk protein | Duration: 24 weeks |
| Mean age: ISP90 61, ISP56 60, cont 61 | Isoflav, mg/day: ISP90 90, ISP56 56, cont nil (AU) | ||
| Soy protein difference: 37 g/day | |||
| Brink 2008 (NL, Italy, France) | Participants: post-men (com) | Design: parallel | DO: int NL 5, It 13, Fr 14, cont NL 5, It 11, Fr 15 |
| Netherlands, Italy, France (Brink et al., 2008) | Analyzed:NL int 45, cont 46; It int 39, cont 39; Fr int 34, cont 34 | Intervention: isoflav ext versus nil | Duration: 52 weeks |
| Mean age:NL int 53, cont 53; It int 53, cont 53; Fr int 54, cont 54 | Isoflav, mg/day: int 110, cont unclear (AU) | ||
| Soy protein difference: 0 g/day | |||
| Brooks 2004 | Participants: post-men (com) | Design: parallel | DO: int 2, cont 0 |
| Canada (Lewis et al., 2006; Brooks et al., 2004) | Analyzed: int 13, cont 15 | Intervention: soy foods versus wheat flour | Duration: 16 weeks |
| Mean age: int 54, cont 53 | Isoflav, mg/day: int 41.4, cont unclear (AU?) | ||
| Soy protein difference: 8 g/day | |||
| Brown 2002 | Participants: pre-men (com) | Design: crossover | DO: 12 |
| USA (Brown et al., 2002) | Analyzed: 14 | Intervention ISP versus alternate foods | Duration: 2 cycles |
| Mean age: 28 | Isoflav, mg/day: int 40, cont none (AU?) | ||
| Soy protein difference: 31 g/day | |||
| Cassidy 1995 | Participants: pre-men (metabolic unit) | Design: crossover | DO: 3 drop outs from study 2 |
| UK (Cassidy et al., 1994, 1995) | Analyzed: Study 1: 6, Study 2: 6, Study 3: 5, Study 4: 6 | Intervention: soy foods versus nil | Duration: 1 cycle |
| Mean age: Study 1: 24, Studies 2–4: unclear | Isoflav, mg/day: Study 1: int 45, cont 1; Study 2: int 25, cont unclear; Study 3: unclear; Study 4: int 23, cont unclear (AU—No) | ||
| Soy protein difference: Study 1 30 g/day; Study 2 7 g/day; Study 3 unclear; Study 4 14 g/day | |||
| Cheng 2007 | Participants: post-men (com) | Design: parallel | DO: 9 (unclear from which arms) |
| Sweden (Cheng et al., 2007) | Analyzed: int 26, cont 24 | Intervention: isoflav ext versus oatmeal | Duration: 12 weeks |
| Mean age: int 58, cont 56 | Isoflav, mg/day: int 60, cont unclear (AU?) | ||
| Soy protein difference: 0 g/day | |||
| Cuevas 2003 | Participants: raised LDL post-men (com) | Design: crossover | DO: unclear |
| Chile (Cuevas et al., 2003) | Analyzed: 18 | Intervention: ISP versus milk protein | Duration: 4 weeks |
| Mean age: 59 | Isoflav, mg/day: int 80, cont unclear (AU?) | ||
| Soy protein difference: 37 g/day | |||
| D’Anna 2007 | Participants: post-men (com) | Design: parallel | DO: 48 int, 37 cont at 2 years |
| Italy (D’Anna et al., 2005; Atteritano et al., 2007; Marini et al., 2007) | Analyzed: int 150, cont 154 | Intervention: isoflav ext versus placebo | Duration: 104 weeks |
| Mean age, sd: int 55, cont 54 | Isoflav, mg/day: int 54, cont nil (AU) | ||
| Soy protein difference: 0 g/day | |||
| Dewell 2002 | Participants: HC post-men (com) | Design: parallel | DO: 4 (unclear from which arms) |
| USA (Dewell et al., 2002; Bruce et al., 2003) | Analyzed: int 22, cont 16 | Intervention: isoflav ext versus placebo | Duration: 26 weeks |
| Mean age: int 69, cont 70 | Isoflav, mg/day: int 90 AU, cont unclear (AU) | ||
| Soy protein difference: 0 g/day | |||
| Duncan 1999 Pre | Participants: pre-men (com) | Design: crossover | DO: 6 |
| USA (Xu et al., 1998; Duncan et al., 1999a; Merz-Demlow et al., 2000; Wangen et al., 2000; Phipps et al., 2001; Fritz et al., 2003) | Analyzed: 14 | Intervention: ISP versus low isoflavone ISP | Duration: 13 weeks |
| Mean age: 27 | Isoflav, mg/day: highISO 128.7, medISO 64.7, Cont 10.0 (AU) | ||
| Soy protein difference: 0 g/day | |||
| Duncan 1999 Post | Participants: post-men (com) | Design: crossover | DO: 4 |
| USA (Duncan et al., 1999b; Xu et al., 2000; Phipps et al., 2001; Wangen et al., 2001) | Analyzed: 18 | Intervention: ISP versus low isoflavone ISP | Duration: 13 weeks |
| Mean age: 57 | Isoflav, mg/day: int 132, cont 7.1 (AU) | ||
| Soy protein difference: 0 g/day | |||
| Gann A 2005 | Participants: pre-men (com) | Design: parallel | DO: unclear |
| USA (Gann et al., 2005) | Analyzed: int 43, cont 43 | Intervention: ISP versus low isoflavone ISP | Duration: 3 cycles |
| Mean age: int 34, cont 33 | Isoflav, mg/day: int 84.4, cont unclear (AU) | ||
| Soy protein difference: 0 g/day | |||
| Gann B 2005 | Participants: pre-men (com) | Design: parallel | DO: unclear |
| USA (Gann et al., 2005) | Analyzed: int 38, cont 30 | Intervention: ISP versus low isoflavone ISP | Duration: 3 cycles |
| Mean age: int 34, cont 33 | Isoflav, mg/day: int 84.4, cont unclear (AU) | ||
| Soy protein difference: 0 g/day | |||
| Gardner 2001 | Participants: HC post-men (com) | Design: parallel | DO: int 3, cont A 2, cont B 1 |
| USA (Gardner et al., 2001) | Analyzed: int 31, cont A 30, cont B 33 | Intervention: ISP versus A milk protein, B low isoflavone ISP | Duration: 12 weeks |
| Mean age: int 63, cont A 58, cont B 58 | Isoflav, mg/day: int 80, contA 2, contB 3 (AU) | ||
| Soy protein difference: 0 or 39 g/day | |||
| Garrido 2006 | Participants: post-men (com) | Design: parallel | DO: none |
| Chile (Garrido et al., 2006) | Analyzed: int 15, cont 14 | Intervention: isoflav ext versus placebo | Duration: 12 weeks |
| Mean age: int 54, cont 53 | Isoflav, mg/day: int 100, cont unclear (AU) | ||
| Soy protein difference: 0 g/day | |||
| Han 2002 | Participants: post-men (com) | Design: parallel | DO: int 1, cont 1 |
| Brazil (Han et al., 2002) | Analyzed: int 40, cont 40 | Intervention: isoflav ext versus glucose | Duration: 16 weeks |
| Mean age: int 48, cont 49 | Isoflav, mg/day: int 100, cont nil (AU) | ||
| Soy protein difference: 0 g/day | |||
| Harkness 2004 | Participants: post-men (com) | Design: crossover | DO:1 |
| USA (Harkness et al., 2004) | Analyzed: 19 | Intervention: isoflav ext versus placebo | Duration: 26 weeks |
| Mean age: 71 | Isoflav, mg/day: int 110, cont unclear (AU - No) | ||
| Soy protein difference: 0 g/day | |||
| Huang 2006 | Participants: post-men (com) | Design: parallel | DO: 1 overall (unclear in which arm) |
| Taiwan (Huang et al., 2006) | Analyzed: int IF200 15, int IF100 15, cont 12 | Intervention: isoflav ext versus nil | Duration: 52 weeks |
| Mean age: IF200 int 52, IF100 int 54, cont 51 | Isoflav, mg/day: IF200 200, IF100 100, cont nil (AU?) | ||
| Soy protein difference: 0 g/day | |||
| Jayagopal 2002 | Participants: diabetic post-men (com) | Design: crossover | DO: 1 |
| UK (Jayagopal et al., 2002) | Analyzed: 32 | Intervention: ISP versus microcrystalline cellulose | Duration: 12 weeks |
| Mean age: 63 | Isoflav, mg/day: int 132, contl unclear (AU?) | ||
| Soy protein difference: 30 g/day | |||
| Knight 2001 | Participants: post-men (com) | Design: parallel | DO: int 3, cont 1 |
| Australia (Knight et al., 2001) | Analyzed: int 9, cont 11 | Intervention: ISP versus milk protein | Duration: 12 weeks |
| Mean age: int 52, cont 54 | Isoflav, mg/day: int 77.4, cont unclear (AU) | ||
| Soy protein difference: 55 g/day | |||
| Kotsopoulos 2000 (PEARL) | Participants: post-men (com) | Design: parallel | DO: int 20?, cont 35? |
| Australia (Kotsopoulos et al., 2000; Teede et al., 2001, 2004, 2005; Dalais et al., 2003) | Analyzed: int 30, cont 20 | Intervention: ISP versus milk protein | Duration: 12 weeks |
| Mean age: int 60, cont 60 | Isoflav, mg/day: int 118, cont unclear (AU?) | ||
| Soy protein difference: 40 g/day | |||
| Kumar 2002 | Participants: pre-men (com) | Design: parallel | DO: int 16, cont 15 |
| USA (Kumar et al., 2002) | Analyzed: int 33, cont 33 | Intervention: ISP versus milk protein | Duration: 3 cycles |
| Mean age: int 41, cont 43 | Isoflav, mg/day: int 40 genistein, cont 0 (AU?) | ||
| Soy protein difference: unclear | |||
| Lichtenstein 2002 | Participants: HC post-men (com, AFP) | Design: crossover | DO: 4 dropped out but were replaced |
| USA (Lichtenstein et al., 2002; Desroches et al., 2004; Wang et al., 2004; Goldin et al., 2005; Vega-Lopez et al., 2005) | Analyzed: 10 or 11 | Intervention: isoflav ext versus nil, also ISP versus low isoflavone ISP | Duration: 6 weeks |
| Mean age: 64 | Isoflav, mg/day: isoflav ext int 114, cont nil, ISP int 102, cont 3 (AU) | ||
| Soy protein difference: g/day | |||
| Mackey 2000 | Participants: HC post-men (com?) | Design: parallel | DO: 5 in total (unclear in which arms) |
| Australia (Eden et al., 2000; Mackey et al., 2000) | Analyzed: int 25, cont 24 | Intervention: ISP versus low isoflavone ISP | Duration: 12 weeks |
| Mean age: int 56, cont 57 | Isoflav, mg/day: int 65, cont <4 (AU?) | ||
| Soy protein difference: 0 g/day | |||
| Martini OC 1999 | Participants: pre-men (com) | Design: crossover | DO: 4 |
| USA (Rutman et al., 1997; Martini et al., 1999) | Analyzed: 16 | Intervention: ISP versus milk protein | Duration: 2 cycles |
| Mean age: 30 | Isoflav, mg/day: int 38, cont unclear (AU?) | ||
| Soy protein difference: 20 g/day | |||
| Maskarinec 2002 | Participants: pre-men (com) | Design: parallel | DO: 5 (unclear in which arms) |
| USA (Maskarinec et al., 2002a, b, 2003) | Analyzed: int 13, cont 16 | Intervention: isoflav ext versus maltodextrin | Duration: 52 weeks |
| Mean age: int 42, cont 43 | Isoflav, mg/day: int 76, cont 0 (AU) | ||
| Soy protein difference: 0 g/day | |||
| Maskarinec 2004 | Participants: pre-men (com) | Design: parallel | DO: 17 int, 14 cont |
| USA (Maskarinec et al., 2004, 2005) | Analyzed: int 92, cont 97 | Intervention: soy foods versus usual diet | Duration: 104 weeks |
| Mean age: int 43, cont 43 | Isoflav, mg/day: int 57.1, cont 7.2 (AU) | ||
| Soy protein difference: 10–44 g/day | |||
| Murkies 1995 | Participants: post-men (com) | Design: parallel | DO: int 5, cont 6 |
| Australia (Murkies et al., 1995) | Analyzed: int 24, cont 23 | Intervention: soy foods versus non-protein cont | Duration: 12 weeks |
| Mean age: int 54, cont 56 | Isoflav, mg/day: unclear (AU?) | ||
| Soy protein difference: 18 g/day | |||
| Nagata 1998 | Participants: pre-men, (com) | Design: parallel | DO: 0 |
| Japan (Nagata et al., 1998; Takatsuka et al., 2000) | Analyzed: int 31, cont 29 | Intervention: soy foods versus usual diet | Duration: 8 weeks |
| Mean age: int 26, cont 27 | Isoflav, mg/day: int 71, cont unclear (AU—No) | ||
| Soy protein difference: 12 g/day | |||
| Nahas 2004 | Participants: post-men (com) | Design: parallel | DO: none |
| Brazil (Nahas et al., 2004) | Analyzed: int 25, cont 25 | Intervention: soy foods versus placebo | Duration: 26 weeks |
| Mean age: int 54, cont 53 | Isoflav, mg/day: int 60, cont unclear (AU?) | ||
| Soy protein difference: 1 g/day | |||
| Nettleton 2004 | Participants: post-men (com) | Design: crossover | DO: 13 |
| USA (Greany et al., 2004, 2008; Nettleton et al., 2004, 2005a, b) | Analyzed: 20 HO br cancer, 20 without | Intervention: ISP versus milk protein | Duration: 6 weeks |
| Mean age: hO br cancer 60, without 56 | Isoflav, mg/day: int 44.4, cont unclear (AU) | ||
| Soy protein difference: 27 g/day | |||
| Nikander 2003 | Participants: post-men HO br cancer (com) | Design: crossover | DO: 6 |
| Finland (Nikander et al., 2003a, b, 2004a, b, 2005; Tormala et al., 2006) | Analyzed: 56 | Intervention: isoflav ext versus placebo | Duration: 12 weeks |
| Mean age: 54 | Isoflav, mg/day: int 114, cont unclear (AU) | ||
| Soy protein difference: 0 g/day | |||
| Scambia 2000 | Participants: post-men (com) | Design: parallel | DO: unclear |
| Italy (Scambia et al., 2000) | Analyzed: int 20, cont 19 | Intervention: isoflav versus placebo | Duration: 6 weeks |
| Mean age: int 54, cont 53 | Isoflav, mg/day: int 50, cont unclear (AU?) | ||
| Soy protein difference: 0 g/day | |||
| Spence 2005 | Participants: post-men (com, AFP) | Design: crossover | DO: 0 |
| USA (Spence et al., 2005) | Analyzed: 15 | Intervention: ISP versus low isoflavone ISP | Duration: 4 wks |
| Mean age: 58 | Isoflav, mg/day: int 65, cont 3.1 (AU) | ||
| Soy protein difference: 0 g/day versus ISP- or 40 g/day versus milk protein | |||
| Squadrito 2002 | Participants: post-men (com) | Design: parallel | DO: 3 int, 4 cont |
| Italy (Squadrito et al., 2002, 2003; Crisafulli et al., 2005; D’Anna et al., 2007) | Analyzed: int 27, cont 26 | Intervention: isoflav ext (genistein) versus placebo | Duration: 52 weeks |
| Mean age: int 56, cont 57 | Isoflav, mg/day: int 54, cont unclear (AU?) | ||
| Soy protein difference: 0 g/day | |||
| Uesugi 2003 | Participants: post-men (com) | Design: parallel | DO: int 0, cont 1 |
| Japan (Uesugi et al., 2003) | Analyzed: int 11, cont 10 | Intervention: isoflav ext versus dextrin | Duration: 12 weeks |
| Mean age: int 55, cont 53 | Isoflav, mg/day: int 61.8, cont unclear (AU—No) | ||
| Soy protein difference: 0 g/day | |||
| Uesugi 2004 | Participants: peri- and post-men (com) | Design: crossover | DO: unclear |
| Japan (Uesugi et al., 2004) | Analyzed: 58 | Intervention: isoflav ext versus placebo | Duration: 4 wks |
| Mean age: 58 | Isoflav, mg/day: int 42, cont unclear (AU) | ||
| Soy protein difference: 0 g/day | |||
| Upmalis 2000 | Participants: post-men, (com) | Design: parallel | DO: 31 int, 24 cont |
| USA (Upmalis et al., 1999, 2000) | Analyzed: int 59, cont 63 | Intervention: isoflav versus placebo | Duration: 12 weeks |
| Mean age: int 55, cont 54 | Isoflav, mg/day: int 50, cont unclear (AU?) | ||
| Soy protein difference: 0 g/day | |||
| Woods 2000 | Participants: post-men (com) | Design: crossover | DO: unclear |
| USA (Woods et al., 2000) | Analyzed: 85 | Intervention: isoflav ext versus placebo | Duration: 12 weeks |
| Mean age: unclear | Isoflav, mg/day: int 45, cont unclear (AU?) | ||
| Soy protein difference: unclear | |||
| Wu 2005 | Participants: post-men, (com, AFP) | Design: parallel | DO: 6 (unclear from which arms) |
| USA (Wu et al., 2005) | Analyzed: int 17, cont 20 | Intervention: soy food versus nil | Duration: 8 weeks |
| Mean age: int 57, cont 60 | Isoflav, mg/day: int 51, cont unclear (AU) | ||
| Soy protein difference: 15 g/day | |||
| Wu 2006 A and B | Participants: post-men (com) | Design: parallel | DO: A int 8, cont 4, B int 1, cont 7 |
| Japan (Wu et al., 2006a, b) | Analyzed: A: int 33, cont 33; B: int 31, cont 31 | Intervention: isoflav ext versus dextrin | Duration: 52 weeks |
| Mean age: A: int 54, cont 55, B: int 54, cont 55 | Isoflav, mg/day: int 75, cont unclear (AU) | ||
| Soy protein difference: 0 g/day | |||
| Zitterman 2004 | Participants: pre-men (com) | Design: crossover | DO: 3 |
| Germany (Zittermann et al., 2004) | Analyzed: 14 | Intervention: soy foods versus nil | Duration: 4 weeks |
| Mean age: 24 | Isoflav, mg/day: int 52, cont <0.1 (AU) | ||
| Soy protein difference: 22 g/day |
ISP, isolated soy protein; AU, aglycone units; Cont, control group; Int, intervention group; Com, community; Isoflav, isoflavone/s; FP, food provided; DO, dropouts; HC, hypercholesterolaemic; Br cancer, breast cancer; HO, history of; AFP, all food provided.
References
- Adams NR. Detection of the effects of phytoestrogens on sheep and cattle. J Anim Sci. 1995;73:1509–1515. doi: 10.2527/1995.7351509x. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H, Mazur W. Phyto-oestrogens and Western diseases. Ann Med. 1997;29:95–120. doi: 10.3109/07853899709113696. [DOI] [PubMed] [Google Scholar]
- Alekel DL, Germain AS, Peterson CT, Hanson KB, Stewart JW, Toda T. Isoflavone-rich soy protein isolate attenuates bone loss in the lumbar spine of perimenopausal women. Am J Clin Nutr. 2000;72:844–852. doi: 10.1093/ajcn/72.3.844. [DOI] [PubMed] [Google Scholar]
- An JP, Tzagarakis-Foster C, Scharschmidt TC, Lomri N, Leitman DC. Estrogen receptor beta-selective transcriptional activity and recruitment of coregulators by phytoestrogens. J Biol Chem. 2001;276:17808–17814. doi: 10.1074/jbc.M100953200. [DOI] [PubMed] [Google Scholar]
- Anon. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008] The Cochrane Collaboration; 2008. www.cochrane-handbook.org . [Google Scholar]
- Arjmandi BH, Khalil DA, Smith BJ, Lucas EA, Juma S, Payton ME, Wild RA. Soy protein has a greater effect on bone in postmenopausal women not on hormone replacement therapy, as evidenced by reducing bone resorption and urinary calcium excretion. J Clin Endocrinol Metab. 2003;88:1048–1054. doi: 10.1210/jc.2002-020849. [DOI] [PubMed] [Google Scholar]
- Arjmandi BH, Khalil DA, Lucas EA, Smith BJ, Sinichi N, Hodges SB, Juma S, Munson ME, Payton ME, Tivis RD, et al. Soy protein may alleviate osteoarthritis symptoms. Phytomedicine. 2004;11:567–575. doi: 10.1016/j.phymed.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Arjmandi BH, Lucas EA, Khalil DA, Devareddy L, Smith BJ, McDonald J, Arquitt AB, Payton ME, Mason C. One year soy protein supplementation has positive effects on bone formation markers but not bone density in postmenopausal women. Nutr J. 2005;4:8. doi: 10.1186/1475-2891-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atteritano M, Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Mazzaferro S, D’Anna R, Cannata ML, Gaudio A, et al. Effects of the phytoestrogen genistein on some predictors of cardiovascular risk in osteopenic, postmenopausal women: a two-year randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2007:3068–3075. doi: 10.1210/jc.2006-2295. [DOI] [PubMed] [Google Scholar]
- Aubertin-Leheudre M, Lord C, Khalil A, Dionne IJ. Effect of 6 months of exercise and isoflavone supplementation on clinical cardiovascular risk factors in obese postmenopausal women: a randomized, double-blind study. Menopause. 2007;14:624–629. doi: 10.1097/gme.0b013e31802e426b. [DOI] [PubMed] [Google Scholar]
- Axelson M, Sjovall J, Gustafsson BE, Setchell KD. Soya—a Dietary source of the non-steroidal estrogen equol in man and animals. J Endocrinol. 1984;102:49–56. doi: 10.1677/joe.0.1020049. [DOI] [PubMed] [Google Scholar]
- Baird DD, Umbach DM, Lansdell L, Hughes CL, Setchell KD, Weinberg CR, Haney AF, Wilcox AJ, McLachlan JA. Dietary intervention study to assess estrogenicity of dietary soy among postmenopausal women. J Clin Endocrinol Metab. 1995;80:1685–1690. doi: 10.1210/jcem.80.5.7745019. [DOI] [PubMed] [Google Scholar]
- Balk E, Chung M, Chew P, Ip S, Raman G, Kupelnick B, Tatsioni A, Sun Y, Devine D, Lau J. Effects of soy on health outcomes. Evid Rep Technol Assess (Summ) 2005;126:1–8. doi: 10.1037/e439502005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum JA, Teng H, Erdman JW, Weigel RM, Klein BP, Persky VW, Freels S, Surya P, Bakhit RM, Ramos E, et al. Long-term intake of soy protein improves blood lipid profiles and increases mononuclear cell low-density-lipoprotein receptor messenger RNA in hypercholesterolemic, postmenopausal women. Am J Clin Nutr. 1998;68:545–551. doi: 10.1093/ajcn/68.3.545. [DOI] [PubMed] [Google Scholar]
- Bennetts HW, Underwood EJ, Shier FL. A specific breeding problem of sheep on subterranean clover pastures in Western Australia. Aust Vet J. 1946;22:2–12. doi: 10.1111/j.1751-0813.1946.tb15473.x. [DOI] [PubMed] [Google Scholar]
- Brink E, Coxam V, Robins S, Wahala K, Cassidy A, Branca F. Long-term consumption of isoflavone-enriched foods does not affect bone mineral density, bone metabolism, or hormonal status in early postmenopausal women: a randomized, double-blind, placebo-controlled study. Am J Clin Nutr. 2008;87:761–770. doi: 10.1093/ajcn/87.3.761. [DOI] [PubMed] [Google Scholar]
- Brooks JD, Ward WE, Lewis JE, Hilditch J, Nickell L, Wong E, Thompson LU. Supplementation with flaxseed alters estrogen metabolism in postmenopausal women to a greater extent than does supplementation with an equal amount of soy. Am J Clin Nutr. 2004;79:318–325. doi: 10.1093/ajcn/79.2.318. [DOI] [PubMed] [Google Scholar]
- Brown BD, Thomas W, Hutchins A, Martini MC, Slavin JL. Types of dietary fat and soy minimally affect hormones and biomarkers associated with breast cancer risk in premenopausal women. Nutr Cancer. 2002;43:22–30. doi: 10.1207/S15327914NC431_2. [DOI] [PubMed] [Google Scholar]
- Bruce B, Messina M, Spiller GA. Isoflavone supplements do not affect thyroid function in iodine-replete postmenopausal women. J Med Food. 2003;6:309–316. doi: 10.1089/109662003772519859. [DOI] [PubMed] [Google Scholar]
- Cassidy A, Bingham SA, Setchell KD. Biological effects of a diet of soy protein rich in isoflavones on the menstrual cycle of premenopausal women. Am J Clin Nutr. 1994;60:333–340. doi: 10.1093/ajcn/60.3.333. [DOI] [PubMed] [Google Scholar]
- Cassidy A, Bingham S, Setchell K. Biological effects of isoflavones in young women: importance of the chemical composition of soyabean products. Br J Nutr. 1995;74:587–601. doi: 10.1079/bjn19950160. [DOI] [PubMed] [Google Scholar]
- Cheng G, Wilczek B, Warner M, Gustafsson JA, Landgren BM. Isoflavone treatment for acute menopausal symptoms. Menopause. 2007;14:468–473. doi: 10.1097/GME.0b013e31802cc7d0. [DOI] [PubMed] [Google Scholar]
- Coon JT, Pittler MH, Ernst E. Trifolium pretense isoflavones in the treatment of menopausal hot flushes: a systematic review and meta-analysis. Phytomedicine. 2007;14:153–159. doi: 10.1016/j.phymed.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Crisafulli A, Altavilla D, Marini H, Bitto A, Cucinotta D, Frisina N, Corrado F, D’Anna R, Squadrito G, Adamo EB, et al. Effects of the phytoestrogen genistein on cardiovascular risk factors in postmenopausal women. Menopause (New York, NY) 2005;12:186–192. doi: 10.1097/00042192-200512020-00013. [DOI] [PubMed] [Google Scholar]
- Cuevas AM, Irribarra VL, Castillo OA, Yanez MD, Germain AM. Isolated soy protein improves endothelial function in postmenopausal hypercholesterolemic women. Eur J Clin Nutr. 2003;57:889–894. doi: 10.1038/sj.ejcn.1601622. [DOI] [PubMed] [Google Scholar]
- D’Anna R, Baviera G, Corrado F, Cancellieri F, Crisafulli A, Squadrito F. The effect of the phytoestrogen genistein and hormone replacement therapy on homocysteine and C-reactive protein level in postmenopausal women. Acta Obstet Gynecol Scand. 2005;84:474–477. doi: 10.1111/j.0001-6349.2005.00661.x. [DOI] [PubMed] [Google Scholar]
- D’Anna R, Cannata ML, Atteritano M, Cancellieri F, Corrado F, Baviera G, Triolo O, Antico F, Gaudio A, Frisina N, et al. Effects of the phytoestrogen genistein on hot flushes, endometrium, and vaginal epithelium in postmenopausal women: a 1-year randomized, double-blind, placebo-controlled study. Menopause. 2007;14:648–655. doi: 10.1097/01.gme.0000248708.60698.98. [DOI] [PubMed] [Google Scholar]
- Dalais FS, Ebeling PR, Kotsopoulos D, McGrath BP, Teede HJ. The effects of soy protein containing isoflavones on lipids and indices of bone resorption in postmenopausal women. Clin Endocrinol. 2003;58:704–709. doi: 10.1046/j.1365-2265.2003.01771.x. [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Higgins JP, Altman DG. Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration; 2008. p. 9.2.3.2. www.cochrane-handbook.org . [Google Scholar]
- Dent SB, Peterson CT, Brace LD, Swain JH, Reddy MB, Hanson KB, Robinson JG, Alekel DL. Soy protein intake by perimenopausal women does not affect circulating lipids and lipoproteins or coagulation and fibrinolytic factors. J Nutr. 2001;131:2280–2287. doi: 10.1093/jn/131.9.2280. [DOI] [PubMed] [Google Scholar]
- Desroches S, Mauger JF, Ausman LM, Lichtenstein AH, Lamarche B. Soy protein favorably affects LDL size independently of isoflavones in hypercholesterolemic men and women. J Nutr. 2004;134:574–579. doi: 10.1093/jn/134.3.574. [DOI] [PubMed] [Google Scholar]
- Dewell A, Hollenbeck CB, Bruce B. The effects of soy-derived phytoestrogens on serum lipids and lipoproteins in moderately hypercholesterolemic postmenopausal women. J Clin Endocrinol Metab. 2002;87:118–121. doi: 10.1210/jcem.87.1.8155. [DOI] [PubMed] [Google Scholar]
- Duncan AM, Merz BE, Xu X, Nagel TC, Phipps WR, Kurzer MS. Soy isoflavones exert modest hormonal effects in premenopausal women. J Clin Endocrinol Metab. 1999;a 84:192–197. doi: 10.1210/jcem.84.1.5387. [DOI] [PubMed] [Google Scholar]
- Duncan AM, Underhill KE, Xu X, Lavalleur J, Phipps WR, Kurzer MS. Modest hormonal effects of soy isoflavones in postmenopausal women [erratum appears in J Clin Endocrinol Metab 2000;85:448] J Clin Endocrinol Metab. 1999;b 84:3479–3484. doi: 10.1210/jcem.84.10.6067. [DOI] [PubMed] [Google Scholar]
- Eden JA, Mackey R, Ekangaki A. Effects of soy protein on postmenopausal women and men with elevated plasma lipids. J Nutr. 2000;130:671S. doi: 10.1002/biof.5520120138. [DOI] [PubMed] [Google Scholar]
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol. 2002;31:140–149. doi: 10.1093/ije/31.1.140. [DOI] [PubMed] [Google Scholar]
- Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- Erdman JW, Jr, Badger TM, Lampe JW, Setchell KD, Messina M. Not All soy products are created equal: caution needed in interpretation of research results. J Nutr. 2004;134:1229S–1233S. doi: 10.1093/jn/134.5.1229S. [DOI] [PubMed] [Google Scholar]
- Follmann D, Elliott P, Suh I, Cutler JA. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 1992;45:769–773. doi: 10.1016/0895-4356(92)90054-q. [DOI] [PubMed] [Google Scholar]
- Fritz KL, Seppanen CM, Kurzer MS, Csallany SA. The in vivo antioxidant activity of soybean isoflavones in human subjects. Nutr Res. 2003;23:479–487. [Google Scholar]
- Gallo D, Zannoni GF, Apollonio P, Martinelli E, Ferlini C, Passetti G, Riva A, Morazzoni P, Bombardelli E, Scambia G. Characterization of the pharmacologic profile of a standardized soy extract in the ovariectomized rat model of menopause: effects on bone, uterus, and lipid profile. Menopause. 2005;12:589–600. doi: 10.1097/01.GME.0000156348.61767.D5. [DOI] [PubMed] [Google Scholar]
- Gann PH, Kazer R, Chatterton R, Gapstur S, Thedford K, Helenowski I, Giovanazzi S, Van Horn L. Sequential, randomized trial of a low-fat, high-fiber diet and soy supplementation: effects on circulating IGF-I and its binding proteins in premenopausal women. Int J Cancer. 2005;116:297–303. doi: 10.1002/ijc.21042. [DOI] [PubMed] [Google Scholar]
- Gardner CD, Newell KA, Cherin R, Haskell WL. The effect of soy protein with or without isoflavones relative to milk protein on plasma lipids in hypercholesterolemic postmenopausal women. Am J Clin Nutr. 2001;73:728–735. doi: 10.1093/ajcn/73.4.728. [DOI] [PubMed] [Google Scholar]
- Garrido A, de la Maza MP, Hirsch S, Valladares L. Soy isoflavones affect platelet thromboxane A2 receptor density but not plasma lipids in menopausal women. Maturitas. 2006;54:270–276. doi: 10.1016/j.maturitas.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Goldin BR, Brauner E, Adlercreutz H, Ausman LM, Lichtenstein AH. Hormonal response to diets high in soy or animal protein without and with isoflavones in moderately hypercholesterolemic subjects. Nutr Cancer. 2005;51:1–6. doi: 10.1207/s15327914nc5101_1. [DOI] [PubMed] [Google Scholar]
- Greany KA, Nettleton JA, Wangen KE, Thomas W, Kurzer MS. Probiotic consumption does not enhance the cholesterol-lowering effect of soy in postmenopausal women. J Nutr. 2004;134:3277–3283. doi: 10.1093/jn/134.12.3277. [DOI] [PubMed] [Google Scholar]
- Greany KA, Nettleton JA, Wangen KE, Thomas W, Kurzer MS. Consumption of isoflavone-rich soy protein does not alter homocysteine or markers of inflammation in postmenopausal women. Eur J Clin Nutr. 2008;62:1419–1425. doi: 10.1038/sj.ejcn.1602885. [DOI] [PubMed] [Google Scholar]
- Han KK, Soares JM, Jr, Haidar MA, de Lima GR, Baracat EC. Benefits of soy isoflavone therapeutic regimen on menopausal symptoms. Obstet Gynecol. 2002;99:389–394. doi: 10.1016/s0029-7844(01)01744-6. [DOI] [PubMed] [Google Scholar]
- Harkness LS, Fiedler K, Sehgal AR, Oravec D, Lerner E. Decreased bone resorption with soy isoflavone supplementation in postmenopausal women. J Womens Health. 2004;13:1000–1007. doi: 10.1089/jwh.2004.13.1000. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes LG, Howes JB, Knight DC. Isoflavone therapy for menopausal flushes: a systematic review and meta-analysis. Maturitas. 2006;55:211. doi: 10.1016/j.maturitas.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Huang HY, Yang HP, Yang HT, Yang TC, Shieh MJ, Huang SY. One-year soy isoflavone supplementation prevents early postmenopausal bone loss but without a dose-dependent effect. J Nutr Biochem. 2006;17:509–517. doi: 10.1016/j.jnutbio.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Hwang CS, Kwak HS, Lim HJ, Lee SH, Kang YS, Choe TB, Hur HG, Han KO. Isoflavone metabolites and their in vitro dual functions: they can act as an estrogenic agonist or antagonist depending on the estrogen concentration. J Steroid Biochem Mol Biol. 2006;101:246–253. doi: 10.1016/j.jsbmb.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Jayagopal V, Albertazzi P, Kilpatrick ES, Howarth EM, Jennings PE, Hepburn DA, Atkin SL. Beneficial effects of soy phytoestrogen intake in postmenopausal women with type 2 diabetes. Diabetes Care. 2002;25:1709–1714. doi: 10.2337/diacare.25.10.1709. [DOI] [PubMed] [Google Scholar]
- Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev. 1993;15:36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- Knight DC, Howes JB, Eden JA, Howes LG. Effects on menopausal symptoms and acceptability of isoflavone-containing soy powder dietary supplementation. Climacteric. 2001;4:13–18. [PubMed] [Google Scholar]
- Kotsopoulos D, Dalais FS, Liang YL, McGrath BP, Teede HJ. The effects of soy protein containing phytoestrogens on menopausal symptoms in postmenopausal women. Climacteric. 2000;3:161–167. doi: 10.1080/13697130008500108. [DOI] [PubMed] [Google Scholar]
- Kuiper G, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Kumar NB, Cantor A, Allen K, Riccardi D, Cox CE. The specific role of isoflavones on estrogen metabolism in premenopausal women. Cancer. 2002;94:1166–1174. doi: 10.1002/cncr.10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey M, Bohday J, Fonseka SM, Ullah AI, Whitehead SA. Dose–response effects of phytoestrogens on the activity and expression of 3beta-hydroxysteroid dehydrogenase and aromatase in human granulosa-luteal cells. J Steroid Biochem Mol Biol. 2005;96:279–286. doi: 10.1016/j.jsbmb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Lethaby AE, Brown J, Morjoribanks J, Kronenberg F, Roberts H, Eden J. Phytoestrogens for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD001395.pub3. CD001395. [DOI] [PubMed] [Google Scholar]
- Lewis JE, Nickell LA, Thompson LU, Szalai JP, Kiss A, Hilditch JR. A randomized controlled trial of the effect of dietary soy and flaxseed muffins on quality of life and hot flashes during menopause. Menopause (New York, NY) 2006;13:631–642. doi: 10.1097/01.gme.0000191882.59799.67. [DOI] [PubMed] [Google Scholar]
- Lichtenstein AH, Jalbert SM, Adlercreutz H, Goldin BR, Rasmussen H, Schaefer EJ, Ausman LM. Lipoprotein response to diets high in soy or animal protein with and without isoflavones in moderately hypercholesterolemic subjects. Arterioscler Thromb Vasc Biol. 2002;22:1852–1858. doi: 10.1161/01.atv.0000033513.18431.a1. [DOI] [PubMed] [Google Scholar]
- Ma DF, Qin LQ, Wang PY, Katoh R. Soy isoflavone intake increases bone mineral density in the spine of menopausal women: meta-analysis of randomized controlled trials. Clin Nutr. 2008;27:57–64. doi: 10.1016/j.clnu.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Mackey R, Ekangaki A, Eden JA. The effects of soy protein in women and men with elevated plasma lipids. Biofactors. 2000;12:251–257. doi: 10.1002/biof.5520120138. [DOI] [PubMed] [Google Scholar]
- Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Atteritano M, Gaudio A, Mazzaferro S, Frisina A, Frisina N, et al. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: a randomized trial. Ann Intern Med. 2007;146:839–847. doi: 10.7326/0003-4819-146-12-200706190-00005. [DOI] [PubMed] [Google Scholar]
- Martini MC, Dancisak BB, Haggans CJ, Thomas W, Slavin JL. Effects of soy intake on sex hormone metabolism in premenopausal women. Nutr Cancer. 1999;34:133–139. doi: 10.1207/S15327914NC3402_2. [DOI] [PubMed] [Google Scholar]
- Maskarinec G, Franke AA, Williams AE, Stanczyk FC. The effects of an isoflavone intervention on the urinary excretion of hormone metabolites in premenopausal women. IARC Sci Pub. 2002;a 156:375–377. [PubMed] [Google Scholar]
- Maskarinec G, Williams AE, Inouye JS, Stanczyk FZ, Franke AA. A randomized isoflavone intervention among premenopausal women. Cancer Epidemiol Biomarkers Prev. 2002;b 11:195–201. [PubMed] [Google Scholar]
- Maskarinec G, Williams AE, Carlin L. Mammographic densities in a one-year isoflavone intervention. Eur J Cancer Prev. 2003;12:165–169. doi: 10.1097/00008469-200304000-00011. [DOI] [PubMed] [Google Scholar]
- Maskarinec G, Franke AA, Williams AE, Hebshi S, Oshiro C, Murphy S, Stanczyk FZ. Effects of a 2-year randomized soy intervention on sex hormone levels in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2004;13:1736–1744. [PubMed] [Google Scholar]
- Maskarinec G, Takata Y, Murphy SP, Franke AA, Kaaks R. Insulin-like growth factor-1 and binding protein-3 in a 2-year soya intervention among premenopausal women. Br J Nutr. 2005;94:362–367. doi: 10.1079/bjn20051525. [DOI] [PubMed] [Google Scholar]
- Merz-Demlow BE, Duncan AM, Wangen KE, Xu X, Carr TP, Phipps WR, Kurzer MS. Soy isoflavones improve plasma lipids in normocholesterolemic, premenopausal women. Am J Clin Nutr. 2000;71:1462–1469. doi: 10.1093/ajcn/71.6.1462. [DOI] [PubMed] [Google Scholar]
- Messina M. The safety and benefits of soybean isoflavones. A natural alternative to conventional hormone therapy? Menopause. 2007;14:958. doi: 10.1097/gme.0b013e31812e5258. [DOI] [PubMed] [Google Scholar]
- Messina M, McCaskill-Stevens W, Lampe JW. Addressing the soy and breast cancer relationship: review, commentary, and workshop proceedings. J Natl Cancer Inst. 2006;a 98:1275–1284. doi: 10.1093/jnci/djj356. [DOI] [PubMed] [Google Scholar]
- Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutr Cancer. 2006;b 55:1–12. doi: 10.1207/s15327914nc5501_1. [DOI] [PubMed] [Google Scholar]
- Moeller LE, Peterson CT, Hanson KB, Dent SB, Lewis DS, King DS, Alekel DL. Isoflavone-rich soy protein prevents loss of hip lean mass but does not prevent the shift in regional fat distribution in perimenopausal women. Menopause. 2003;10:322–331. doi: 10.1097/01.GME.0000054763.94658.FD. [DOI] [PubMed] [Google Scholar]
- Murkies AL, Lombard C, Strauss BJ, Wilcox G, Burger HG, Morton MS. Dietary flour supplementation decreases post-menopausal hot flushes: effect of soy and wheat. Maturitas. 1995;21:189–195. doi: 10.1016/0378-5122(95)00899-v. [DOI] [PubMed] [Google Scholar]
- Nagata C, Takatsuka N, Inaba S, Kawakami N, Shimizu H. Effect of soymilk consumption on serum estrogen concentrations in premenopausal women. J Natl Cancer Inst. 1998;90:1830–1835. doi: 10.1093/jnci/90.23.1830. [DOI] [PubMed] [Google Scholar]
- Nahas EP, Nahas Neto J, DeLuca L, Traiman P, Pontes A, Dalben I. Benefits of soy germ isoflavone in postmenopausal women with contraindication of conventional hormone replacement therapy. Maturitas. 2004;48:372–380. doi: 10.1016/j.maturitas.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Nelson HD, Vesco KK, Haney E, Fu R, Nedrow A, Miller J, Nicolaidis C, Walker M, Humphrey L. Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA. 2006;295:2057–2071. doi: 10.1001/jama.295.17.2057. [DOI] [PubMed] [Google Scholar]
- Nettleton JA, Greany KA, Thomas W, Wangen KE, Adlercreutz H, Kurzer MS. Plasma phytoestrogens are not altered by probiotic consumption in postmenopausal women with and without a history of breast cancer. J Nutr. 2004;134:1998–2003. doi: 10.1093/jn/134.8.1998. [DOI] [PubMed] [Google Scholar]
- Nettleton JA, Greany KA, Thomas W, Wangen KE, Adlercreutz H, Kurzer MS. The effect of soy consumption on the urinary 2:16-hydroxyestrone ratio in postmenopausal women depends on equol production status but is not influenced by probiotic consumption. J Nutr. 2005;a 135:603–608. doi: 10.1093/jn/135.3.603. [DOI] [PubMed] [Google Scholar]
- Nettleton JA, Greany KA, Thomas W, Wangen KE, Adlercreutz H, Kurzer MS. Short-term soy and probiotic supplementation does not markedly affect concentrations of reproductive hormones in postmenopausal women with and without histories of breast cancer. J Altern Complement Med (New York, N Y) 2005;b 11:1067–1074. doi: 10.1089/acm.2005.11.1067. [DOI] [PubMed] [Google Scholar]
- Nikander E, Kilkkinen A, Metsa-Heikkila M, Adlercreutz H, Pietinen P, Tiitinen A, Ylikorkala O. A randomized placebo-controlled crossover trial with phytoestrogens in treatment of menopause in breast cancer patients. Obstet Gynecol. 2003;a 101:1213–1220. doi: 10.1016/s0029-7844(03)00232-1. [DOI] [PubMed] [Google Scholar]
- Nikander E, Metsa-Heikkila M, Tiitinen A, Ylikorkala O. Evidence of a lack of effect of a phytoestrogen regimen on the levels of C-reactive protein, E-selectin, and nitrate in postmenopausal women. J Clin Endocrinol Metab. 2003;b 88:5180–5185. doi: 10.1210/jc.2003-030362. [DOI] [PubMed] [Google Scholar]
- Nikander E, Metsa-Heikkila M, Ylikorkala O, Tiitinen A. Effects of phytoestrogens on bone turnover in postmenopausal women with a history of breast cancer. J Clin Endocrinol Metab. 2004;a 89:1207–1212. doi: 10.1210/jc.2003-031166. [DOI] [PubMed] [Google Scholar]
- Nikander E, Tiitinen A, Laitinen K, Tikkanen M, Ylikorkala O. Effects of isolated isoflavonoids on lipids, lipoproteins, insulin sensitivity, and ghrelin in postmenopausal women. J Clin Endocrinol Metab. 2004;b 89:3567–3572. doi: 10.1210/jc.2003-032229. [DOI] [PubMed] [Google Scholar]
- Nikander E, Rutanen EM, Nieminen P, Wahlstrom T, Ylikorkala O, Tiitinen A. Lack of effect of isoflavonoids on the vagina and endometrium in postmenopausal women. Fertil Steril. 2005;83:137–142. doi: 10.1016/j.fertnstert.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Persky VW, Turyk ME, Wang L, Freels S, Chatterton R, Jr, Barnes S, Erdman J, Jr, Sepkovic DW, Bradlow HL, Potter S. Effect of soy protein on endogenous hormones in postmenopausal women [erratum appears in Am J Clin Nutr 2002;76:695] Am J Clin Nutr. 2002;75:145–153. doi: 10.1093/ajcn/75.1.145. [DOI] [PubMed] [Google Scholar]
- Phipps WR, Wangen KE, Duncan AM, Merz-Demlow BE, Xu X, Kurzer MS. Lack of effect of isoflavonic phytoestrogen intake on leptin concentrations in premenopausal and postmenopausal women. Fertil Steril. 2001;75:1059–1064. doi: 10.1016/s0015-0282(01)01777-0. [DOI] [PubMed] [Google Scholar]
- Pike ACW, Brzozowski AM, Hubbard RE, Bonn T, Thorsell AG, Engstrom O, Ljunggren J, Gustafsson JK, Carlquist M. Structure of the ligand-binding domain of oestrogen receptor beta in the presence of a partial agonist and a full antagonist. EMBO J. 1999;18:4608–4616. doi: 10.1093/emboj/18.17.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter SM, Baum JA, Teng H, Stillman RJ, Shay NF, Erdman JW., Jr Soy protein and isoflavones: their effects on blood lipids and bone density in postmenopausal women. Am J Clin Nutr. 1998;68:1375S–1379S. doi: 10.1093/ajcn/68.6.1375S. [DOI] [PubMed] [Google Scholar]
- Rice S, Mason HD, Whitehead SA. Phytoestrogens and their low dose combinations inhibit mRNA expression and activity of aromatase in human granulosa-luteal cells. J Steroid Biochem Mol Biol. 2006;101:216–225. doi: 10.1016/j.jsbmb.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Rosselli M, Rienhart K, Imthurn B, Keller PJ, Dubey RK. Cellular and biochemical mechanisms by which environmental oestrogens influence reproductive function. Hum Reprod Update. 2000;6:332–350. doi: 10.1093/humupd/6.4.332. [DOI] [PubMed] [Google Scholar]
- Rutman JY, Dancisak BB, Nagel TC, Martini MC, Slavin JL. Soy intake and oral contraceptives: effects on sex hormones and the menstrual cycle [abstract] Am J Clin Nutr. 1997;66:214. [Google Scholar]
- Sacks FM, Lichtenstein AH, Van Horn L, Harris W, Kris-Etherton PM, Winston MC. Soy protein, isoflavones, and cardiovascular health: a summary of a statement for professionals from the American heart association nutrition committee. Arterioscler Thromb Vasc Biol. 2006;26:1689–1692. doi: 10.1161/01.ATV.0000227471.00284.ef. [DOI] [PubMed] [Google Scholar]
- Scambia G, Mango D, Signorile PG, Angeli RA, Palena C, Gallo D, Bombardelli E, Morazzoni P, Riva A, Mancuso S. Clinical effects of a standardized soy extract in postmenopausal women: a pilot study. Menopause. 2000;7:105–111. doi: 10.1097/00042192-200007020-00006. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J Nutr. 1999;129:758S–767S. doi: 10.1093/jn/129.3.758S. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Borriello SP, Hulme P, Kirk DN, Axelson M. Nonsteroidal estrogens of dietary origin—possible roles in hormone-dependent disease. Am J Clin Nutr. 1984;40:569–578. doi: 10.1093/ajcn/40.3.569. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Gosselin SJ, Welsh MB, Johnston JO, Balistreri WF, Kramer LW, Dresser BL, Tarr MJ. Dietary estrogens—a probable cause of infertility and liver disease in captive cheetahs. Gastroenterology. 1987;93:225–233. doi: 10.1016/0016-5085(87)91006-7. [DOI] [PubMed] [Google Scholar]
- Spence LA, Lipscomb ER, Cadogan J, Martin B, Wastney ME, Peacock M, Weaver CM. The effect of soy protein and soy isoflavones on calcium metabolism in postmenopausal women: a randomized crossover study. Am J Clin Nutr. 2005;81:916–922. doi: 10.1093/ajcn/81.4.916. [DOI] [PubMed] [Google Scholar]
- Squadrito F, Altavilla D, Morabito N, Crisafulli A, D’Anna R, Corrado F, Ruggeri P, Campo GM, Calapai G, Caputi AP, et al. The effect of the phytoestrogen genistein on plasma nitric oxide concentrations, endothelin-1 levels and endothelium dependent vasodilation in postmenopausal women. Atherosclerosis. 2002;163:339–347. doi: 10.1016/s0021-9150(02)00013-8. [DOI] [PubMed] [Google Scholar]
- Squadrito F, Altavilla D, Crisafulli A, Saitta A, Cucinotta D, Morabito N, D’Anna R, Corrado F, Ruggeri P, Frisina N, et al. Effect of genistein on endothelial function in postmenopausal women: a randomized, double-blind, controlled study. Am J Med. 2003;114:470–476. doi: 10.1016/s0002-9343(03)00059-7. [DOI] [PubMed] [Google Scholar]
- St Germain A, Peterson CT, Robinson JG, Alekel DL. Isoflavone-rich or isoflavone-poor soy protein does not reduce menopausal symptoms during 24 weeks of treatment. Menopause. 2001;8:17–26. doi: 10.1097/00042192-200101000-00005. [DOI] [PubMed] [Google Scholar]
- Swain JH, Alekel DL, Dent SB, Peterson CT, Reddy MB. Iron indexes and total antioxidant status in response to soy protein intake in perimenopausal women. Am J Clin Nutr. 2002;76:165–171. doi: 10.1093/ajcn/76.1.165. [DOI] [PubMed] [Google Scholar]
- Takatsuka N, Nagata C, Kurisu Y, Inaba S, Kawakami N, Shimizu H. Hypocholesterolemic effect of soymilk supplementation with usual diet in premenopausal normolipidemic Japanese women. Prev Med. 2000;31:308–314. doi: 10.1006/pmed.2000.0714. [DOI] [PubMed] [Google Scholar]
- Teede HJ, Dalais FS, Kotsopoulos D, Liang YL, Davis S, McGrath BP. Dietary soy has both beneficial and potentially adverse cardiovascular effects: a placebo-controlled study in men and postmenopausal women. J Clin Endocrinol Metab. 2001;86:3053–3060. doi: 10.1210/jcem.86.7.7645. [DOI] [PubMed] [Google Scholar]
- Teede HJ, Dalais FS, McGrath BP. Dietary soy containing phytoestrogens does not have detectable estrogenic effects on hepatic protein synthesis in postmenopausal women. Am J Clin Nutr. 2004;79:396–401. doi: 10.1093/ajcn/79.3.396. [DOI] [PubMed] [Google Scholar]
- Teede HJ, Dalais FS, Kotsopoulos D, McGrath BP, Malan E, Gan TE, Peverill RE. Dietary soy containing phytoestrogens does not activate the hemostatic system in postmenopausal women. J Clin Endocrinol Metab. 2005 doi: 10.1210/jc.2004-1428. [DOI] [PubMed] [Google Scholar]
- Tormala RM, Nikander E, Tiitinen A, Vaisanen-Tommiska M, Ylikorkala O, Mikkola TS. Serum cholesterol efflux potential in postmenopausal women treated with isolated isoflavones. Menopause. 2006;13:96–101. doi: 10.1097/01.gme.0000191210.13115.90. [DOI] [PubMed] [Google Scholar]
- Uesugi T, Toda T, Okuhira T, Chen JT. Evidence of estrogenic effect by the three-month-intervention of isoflavone on vaginal maturation and bone metabolism in early postmenopausal women. Endocr J. 2003;50:613–619. doi: 10.1507/endocrj.50.613. [DOI] [PubMed] [Google Scholar]
- Uesugi S, Watanabe S, Ishiwata N, Uehara M, Ouchi K. Effects of isoflavone supplements on bone metabolic markers and climacteric symptoms in Japanese women. Biofactors. 2004;22:221–228. doi: 10.1002/biof.5520220145. [DOI] [PubMed] [Google Scholar]
- Upmalis DH, Wiita B, Cone FL, Lamia CA, Warren M, Bradley L. Improvement of menopausal symptoms and quality of life by oral soy extract tablets. J Nutr. 1999;130:707S. [Google Scholar]
- Upmalis DH, Lobo R, Bradley L, Warren M, Cone FL, Lamia CA. Vasomotor symptom relief by soy isoflavone extract tablets in postmenopausal women: a multicenter, double-blind, randomized, placebo-controlled study. Menopause (New York, NY) 2000;7:236–242. doi: 10.1097/00042192-200007040-00005. [DOI] [PubMed] [Google Scholar]
- Vega-Lopez S, Yeum K-J, Lecker JL, Ausman LM, Johnson EJ, Devaraj S, Jialal I, Lichtenstein AH. Plasma antioxidant capacity in response to diets high in soy or animal protein with or without isoflavones. Am J Clin Nutr. 2005;81:43–49. doi: 10.1093/ajcn/81.1.43. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jones PJ, Ausman LM, Lichtenstein AH. Soy protein reduces triglyceride levels and triglyceride fatty acid fractional synthesis rate in hypercholesterolemic subjects. Atherosclerosis. 2004;173:269–275. doi: 10.1016/j.atherosclerosis.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Wangen KE, Duncan AM, Merz-Demlow BE, Xu X, Marcus R, Phipps WR, Kurzer MS. Effects of soy isoflavones on markers of bone turnover in premenopausal and postmenopausal women. J Clin Endocrinol Metab. 2000;85:3043–3048. doi: 10.1210/jcem.85.9.6787. [DOI] [PubMed] [Google Scholar]
- Wangen KE, Duncan AM, Xu X, Kurzer MS. Soy isoflavones improve plasma lipids in normocholesterolemic and mildly hypercholesterolemic postmenopausal women. Am J Clin Nutr. 2001;73:225–231. doi: 10.1093/ajcn/73.2.225. [DOI] [PubMed] [Google Scholar]
- Williamson-Hughes PS, Flickinger BD, Messina M, Empie MW. Isoflavone supplements containing predominantly genistein reduce hot flash symptoms: a critical review of published studies. Menopause. 2006;13:831–839. doi: 10.1097/01.gme.0000227330.49081.9e. [DOI] [PubMed] [Google Scholar]
- Woods M, Speigelman D, Hertzmark E, LaBrode A, Longcope C. Dietary soy supplement and menopausal hormones and hot flashes. J Nutr. 2000;130:671S. [Google Scholar]
- Wu AH, Stanczyk FZ, Martinez C, Tseng CC, Hendrich S, Murphy P, Chaikittisilpa S, Stram D, Pike MC. A controlled 2-mo dietary fat reduction and soy food supplementation study in postmenopausal women. Am J Clin Nutr. 2005;81:1133–1141. doi: 10.1093/ajcn/81.5.1133. [DOI] [PubMed] [Google Scholar]
- Wu J, Oka J, Higuchi M, Tabata I, Toda T, Fujioka M, Fuku N, Teramoto T, Okuhira T, Ueno T, et al. Cooperative effects of isoflavones and exercise on bone and lipid metabolism in postmenopausal Japanese women: a randomized placebo-controlled trial. Metabolism. 2006;a 55:423–433. doi: 10.1016/j.metabol.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Wu J, Oka J, Tabata I, Higuchi M, Toda T, Fuku N, Ezaki J, Sugiyama F, Uchiyama S, Yamada K, et al. Effects of isoflavone and exercise on BMD and fat mass in postmenopausal Japanese women: a 1-year randomized placebo-controlled trial. J Bone Miner Res. 2006;b 21:780–789. doi: 10.1359/jbmr.060208. [DOI] [PubMed] [Google Scholar]
- Wu AH, Yu MC, Tseng C-C, Pike MC. Epidemiology of soy exposure and breast cancer risk. Br J Cancer. 2008;98:9–14. doi: 10.1038/sj.bjc.6604145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Duncan AM, Merz BE, Kurzer MS. Effects of soy isoflavones on estrogen and phytoestrogen metabolism in premenopausal women. Cancer Epidemiol Biomarkers Prev. 1998;7:1101–1108. [PubMed] [Google Scholar]
- Xu X, Duncan AM, Wangen KE, Kurzer MS. Soy consumption alters endogenous estrogen metabolism in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2000;9:781–786. [PubMed] [Google Scholar]
- Zittermann A, Geppert J, Baier S, Zehn N, Gouni-Berthold I, Berthold HK, Reinsberg J, Stehle P. Short-term effects of high soy supplementation on sex hormones, bone markers, and lipid parameters in young female adults. Eur J Nutr. 2004;43:100–108. doi: 10.1007/s00394-004-0447-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.