Abstract
BACKGROUND:
Facilitated percutaneous coronary intervention (PCI) is defined as the administration of fibrinolytic therapy and/or glycoprotein (GP) IIb/IIIa inhibitors to minimize myocardial ischemia time while waiting for PCI. A pooled meta-analysis suggested that facilitated PCI was associated with higher rates of mortality and morbidity compared with nonfacilitated PCI.
OBJECTIVE:
The heterogeneous and complex trials of facilitated PCI were systematically reviewed to identify where this strategy may be beneficial and deserving of further research.
METHODS:
MEDLINE, EMBASE, the Cochrane database, the Internet and conference proceedings were searched to obtain relevant trials. Human studies that randomly assigned patients to fibrinolytic-facilitated PCI (administration of fibrinolytic therapy alone or in combination with GP IIb/IIIa inhibitors before angiography) versus nonfacilitated PCI were included.
RESULTS:
Nine trials encompassing 3836 patients were reviewed. The facilitated PCI strategy was fibrinolytic therapy alone in seven trials and half-dose fibrinolytic therapy plus GP IIb/IIIa inhibitors in two trials. In patients who had fibrinolysis less than 2 h after symptom onset (mainly in the prehospital setting) and/or slightly delayed PCI 3 h to 24 h after fibrinolysis, facilitated PCI was associated with the greatest Thrombolysis In Myocardial Infarction (TIMI) grade 3 flow and a trend toward reduced mortality. Overall, facilitated PCI was associated with increased intracranial hemorrhage and reinfarction. Combining half-dose fibrinolytic therapy and GP IIb/IIIa inhibitors reduced reinfarction but increased major bleeding.
CONCLUSIONS:
Facilitated PCI cannot be recommended outside of experimental protocols at this time. Further research should focus on selecting patients with higher benefit-to-risk ratios and performing prehospital fibrinolysis with optimal antiplatelet or antithrombin therapy, as well as slightly delayed PCI in patients who are stable or geographically removed from PCI facilities.
Keywords: Angioplasty, Clinical trials, Fibrinolysis, Health care delivery, Myocardial infarction, Thrombolysis
Abstract
HISTORIQUE :
L’intervention coronarienne percutanée (ICP) facilitée désigne l’administration d’une thérapie fibrinolytique ou des inhibiteurs de la glycoprotéine (GP) IIb/IIIa pour réduire au minimum le temps d’ischémie myocardique dans l’attente d’une ICP. Selon une méta-analyse groupée, l’ICP facilitée s’associait à des taux plus élevés de mortalité et de morbidité que l’ICP non facilitée.
OBJECTIF :
Les essais hétérogènes et complexes de l’ICP facilitée ont fait l’objet d’un examen systématique pour repérer en quoi cette stratégie peut être bénéfique et digne d’intérêt dans le cadre de recherches plus approfondies.
MÉTHODOLOGIE :
Les auteurs ont fait des recherches dans les bases de données MEDLINE, EMBASE et de la bibliothèque Cochrane, Internet et les comptes rendus de congrès pour trouver les essais pertinents. Ils ont inclus des essais sur des humains dans lesquels on avait réparti des patients de manière aléatoire entre l’ICP facilitée par une thérapie fibrinolytique (administration de la thérapie fibrinolytique seule ou en association avec des inhibiteurs de la GP IIb/IIIa avant l’angiographie) et l’ICP non facilitée.
RÉSULTATS :
Les auteurs ont passé en revue neuf essais portant sur 3 836 patients. Sept essais privilégiaient la stratégie d’ICP facilitée par thérapie fibrinolytique seule, tandis que deux portaient sur une demi-dose de thérapie fibrinolytique associée à des inhibiteurs de la GP IIb/IIIa. Chez les patients qui présentaient une fibrinolyse moins de deux heures après l’apparition des symptômes (surtout avant l’hospitalisation) ou un léger report de l’ICP de trois à 24 heures après la fibrinolyse, l’ICP facilitée était reliée à la meilleure thrombolyse dans l’infarctus du myocarde (TIMI) avec débit de grade 3 et à une tendance vers une réduction de la mortalité. Dans l’ensemble, l’ICP facilitée s’associait à un accroissement des hémorragies intracrâniennes et des nouveaux infarctus. L’association d’une demi-dose de thérapie fibrinolytique et d’inhibiteurs de la GP IIb/IIIa réduisait le nombre de nouveaux infarctus, mais accroissait le nombre de graves hémorragies.
CONCLUSIONS :
Pour l’instant, l’ICP facilitée ne peut être recommandée hors des protocoles expérimentaux. Des recherches devraient porter sur la sélection de patients aux ratios plus élevés entre les avantages et les risques et sur la fibrinolyse préhospitalière avec thérapie antiplaquettaire ou antithrombotique optimale, ainsi que sur un léger report de l’ICP chez des patients stables ou habitant loin des installations d’ICP.
Primary percutaneous coronary intervention (PCI) has been shown to be superior to fibrinolytic therapy for the treatment of ST segment elevation myocardial infarction (STEMI) (1). However, primary PCI is suboptimal when there are prolonged delays for interhospital transfer or resource mobilization. Facilitated PCI is defined as the administration of fibrinolytic therapy and/or glycoprotein (GP) IIb/IIIa inhibitors while waiting for PCI. The rationale is to open infarct-related arteries (IRAs) as soon as possible, minimize myocardial ischemia time and, thus, salvage at-risk myocardium. Despite the theoretical benefits, randomized controlled trials have failed to support the clinical benefits of this strategy. These trials varied substantially in terms of facilitating regimens, timing of fibrinolysis, timing of PCI, and coadministered antiplatelet or antithrombin therapies. A recent meta-analysis (2) showed that facilitated PCI was associated with increased short-term mortality, reinfarction, major bleeding and stroke. Although the results of this meta-analysis were unequivocally negative, a systematic review may be better suited to address these heterogeneous and complex trials. Our objective was to systematically review trials of fibrinolytic-facilitated versus nonfacilitated PCI, and determine when this strategy may be beneficial and deserving of further research.
METHODS
MEDLINE, EMBASE and the Cochrane database were searched from their respective inceptions to 2006 to identify published randomized controlled trials of fibrinolytic-facilitated PCI versus nonfacilitated PCI. Search terms included ‘myocardial infarction’, ‘fibrinolytic agents’, ‘thrombolytic therapy’, ‘tissue plasminogen activator’, ‘streptokinase’, ‘alteplase’, ‘reteplase’, ‘tenecteplase’, ‘percutaneous transluminal coronary angioplasty’, ‘randomized controlled trials’, ‘clinical trials’, ‘randomized’, ‘facilitated’ and ‘percutaneous coronary intervention’. Abstracts from cardiology conferences, references from retrieved publications, as well as the Internet (www.clinicaltrials.gov, www.clinicaltrialresults.com, www.theheart.org, www.medscape.com), were also searched to identify unpublished trials. Furthermore, investigators were contacted to obtain additional information on ongoing and completed trials. Human studies in English and French were included.
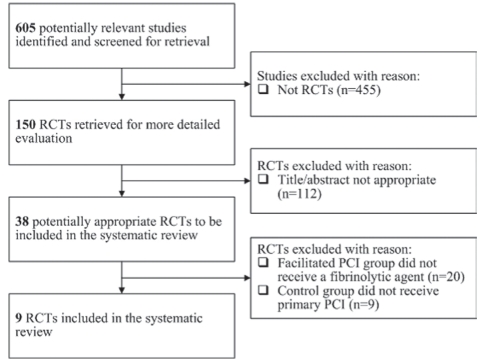
Facilitated PCI was defined as the administration of fibrinolytic therapy alone or in combination with GP IIb/IIIa inhibitors before planned angiography and PCI if indicated. Nonfacilitated PCI was defined as planned angiography and primary PCI if indicated without the use of fibrinolytic therapy. Because primary PCI has been shown to be superior to fibrinolytic therapy, the objective of the present study was to determine whether facilitated PCI was superior to nonfacilitated PCI. Trials of facilitated PCI versus fibrinolytic therapy alone were therefore excluded because they did not address the research question. The search identified 605 studies, 150 of which were randomized controlled trials. Based on the titles and abstracts, 38 trials were retrieved for detailed evaluation. Nine trials met the prespecified inclusion criteria and are reviewed in the present systematic review. The Quality Of Reporting Of Meta-analyses flow diagram, as shown in Figure 1, outlines the process of including and excluding potentially relevant trials.
Figure 1).
The Quality Of Reporting Of Meta-analyses flow diagram. PCI Percutaneous coronary intervention; RCTs Randomized controlled trials
Data were extracted in duplicate by two investigators and verified independently by a third investigator. Disagreements were resolved by consensus. The following study variables were extracted: inclusion and exclusion criteria, number of patients, baseline characteristics, reperfusion time delays, facilitating regimens and cointerventions. The following outcome measures were extracted: mortality, reinfarction, need for reintervention, major adverse cardiac events (MACE), congestive heart failure, cardiogenic shock, infarct size, left ventricular ejection fraction (LVEF), Thrombolysis In Myocardial Infarction (TIMI) grade 3 flow at and after initial angiography, TIMI blush score, ST segment resolution, stroke, intracranial hemorrhage, major bleeding and transfusion requirements. Predetermined and post hoc subgroup analyses were explored in detail to detect confounding variables, effect modifiers and clinical scenarios in which facilitated PCI may be beneficial.
IRA patency was assessed according to the TIMI flow score (3), which has been used extensively in clinical trials. The TIMI flow score is divided into four grades: grade 0 appears as no flow beyond the occlusion; grade 1 appears as slow flow beyond the occlusion, but the contrast fails to opacify the entire distal coronary bed; grade 2 appears as slowed passage and clearance of contrast distal to the occlusion; and grade 3 appears as rapid passage of contrast distal to the occlusion. Microvascular blood flow was assessed according to the TIMI blush score. The TIMI blush score is an indicator of myocardial perfusion and microvascular blood flow, which has been shown to be additive to indicators of macrovascular blood flow such as the TIMI flow score (4).
RESULTS
PCI facilitated by fibrinolytic therapy alone
Fibrinolytic agents exert their effect by cleaving the Arg560-Val561 bond of plasminogen to generate the active enzyme plasmin (5). These agents have been expanded from primary reperfusion agents to facilitating reperfusion agents. In the era of intracoronary stents, fibrinolysis followed by routine early PCI has been shown to be superior to fibrinolysis followed by conservative management (6,7). It is less clear whether fibrinolysis followed by immediate or early PCI is superior to direct primary PCI. Tables 1 through 4 summarize the trials addressing this issue (seven trials of PCI facilitated by fibrinolytic therapy alone and two trials of PCI facilitated by half-dose fibrinolytic therapy plus GP IIb/IIIa inhibitors).
TABLE 1.
Characteristics of trials using fibrinolytics alone for facilitated percutaneous coronary intervention (PCI)
| SANI (8) 1992 | LIMI (11) 1999 | PACT (10) 1999 | PRAGUE-1 (9) 2000 | ASSENT-4 (14) 2006 | GRACIA-2 (12) 2007 | WEST (13) 2006 | |
|---|---|---|---|---|---|---|---|
|
Study design | |||||||
| Participants, n | 122 | 224 | 606 | 300 | 1667 | 212 | 304 |
| Age, years (mean ± SD) | 57±10 | 59±12 | 58±10 | 62±12 | 61±12.1 | 63±13 | 58 (IQR 50–69) |
| Female sex, % | 17 | 19 | 21 | 28 | 23 | 19 | 20 |
| Anterior territory, % | 44 | 36 | 37 | 51 | 48 | 47 | 40 |
| Facilitated PCI (Fac) | Full-dose SK + PCI | Full-dose rt-PA + transfer for PCI | Variable dose rt-PA + PCI | Full-dose SK + transfer for PCI | Full-dose TNK + PCI | Full-dose TNK + PCI | Full-dose TNK + PCI |
| Nonfacilitated PCI (Nonfac) | Placebo + PCI | Transfer for PCI | Placebo + PCI | Transfer for PCI | PCI | PCI | Clopidogrel + PCI |
| Time delays | Fac | Nonfac | Fac | Nonfac | Fac | Nonfac | Fac | Nonfac | Fac | Nonfac | Fac | Nonfac | Fac | Nonfac |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Symptom-to-randomization, min (median) | 69 | 83 | 140 | 130 | 84 | 84 | 127 | 135 | 140* | 135* | 191 | 192 | 114† | 100† |
| Randomization-to-drug, min (median) | 91 | – | 10 | – | 49 | – | 25 | – | 10 | – | N/R | – | 16 | – |
| Drug-to-angiography, min (median) | 31 | – | 90 | – | 49 | – | 68 | – | 104 | – | N/R | – | 295 | – |
| Randomization-to-angiography, min (median) | 121 | 116 | 100 | 85 | 98 | 98 | 93 | 80 | 115 | 107 | 353 | 65 | 311 | 76 |
| Concomitant therapies | Fac | Nonfac | Fac | Nonfac | Fac | Nonfac | Fac | Nonfac | Fac | Nonfac | Fac | Nonfac | Fac | Nonfac |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylsalicylic acid | All | All | All | All | All | All | All | All | All | All | 89% | 91% | All | All |
| Heparin | 10,000 U | 10,000 U | 5000 U | 10,000 U | 5000 U + infusion | 5000 U + infusion | Fraxiparin | 10,000 U + fraxiparin | 60 U/kg (max 4000 U) | 70 U/kg | Enoxaparin | ACT 350 s – 450 s | Enoxaparin (no bolus) | Enoxaparin |
| Intracoronary stents | 0 | If suboptimal PCTA | 26% | If suitable | 81%–86% | 96% | 98% | 97% | ||||||
| Thienopyridines post-PCI | None | None | MD choice | MD choice | None | None | All (ticlopidine) | All (ticlopidine) | 87% | 91% | 91% | All stents | All | |
| GP IIb/IIIa inhibitors post-PCI | None | None | None | None | 5% | 5% | None | None | 13% | 50% | 25% | 91% | 48% | 97% |
Symptom-to-randomization time by site of randomization – PCI hospital, 160 min; non-PCI hospital, 130 min – 135 min; and ambulance, 105 min;
Symptom-to-randomization time by site of randomization – in-hospital (60%–63% of patients), 125 min – 135 min; prehospital (37%–40% of patients), 72 min – 76 min. ACT Activated clotting time; GP Glycoprotein; IQR Interquartile range; max Maximum; MD Medical doctor; N/R Not reported; PTCA Percutaneous transluminal coronary angioplasty; rt-PA Alteplase; SK Streptokinase; TNK Tenecteplase
TABLE 4.
Results of trials using combination fibrinolytics and glycoprotein IIb/IIIa inhibitors for facilitated percutaneous coronary intervention (PCI)
|
BRAVE (16) 2004 |
ADVANCE MI (15) 2005 |
|||
|---|---|---|---|---|
| Fac | Nonfac | Fac | Nonfac | |
| Angiographic outcomes | ||||
| TIMI-3 flow at initial angiography, % | 40* | 18* | 41* | 21* |
| TIMI-3 flow after PCI, % | 87 | 87 | N/R | N/R |
| TIMI blush score | Mean 2.4 | Mean 3.1 | 2–3: 30% | 2–3: 20% |
| LVEF (ventriculogram), % | 52 | 52 | N/R | N/R |
| Clinical outcomes, % | ||||
| Mortality† | 1.6 | 1.6 | 6.8* | 0.0* |
| Reinfarction | 0.8 | 0 | 1.4 | 2.7 |
| Reintervention | 0.8 | 0 | N/R | N/R |
| Congestive heart failure | N/R | N/R | 6.8 | 1.4 |
| MACE‡ | 6.4 | 4.7 | 12.2* | 2.7* |
| Safety outcomes, % | ||||
| Any stroke | 0.8 | 0 | N/R | N/R |
| Intracranial hemorrhage | 0.8 | 0 | N/R | N/R |
| Major bleeding episode | 5.6 | 1.6 | 14.9* | 8.1* |
| Transfusion requirement | 3.2 | 0.8 | N/R | N/R |
Significant difference P<0.05;
Mortality – BRAVE: 30-day; ADVANCE MI: 30-day;
Major adverse cardiac events (MACE) – BRAVE: 6-month mortality or reinfarction or stroke; ADVANCE MI: 30-day death or congestive heart failure or cardiogenic shock. Fac Facilitated PCI; LVEF Left ventricular ejection fraction; N/R Not reported; Nonfac Nonfacilitated PCI; TIMI-3 Thrombolysis In Myocardial Infarction grade 3
The seven trials of PCI facilitated by fibrinolytic therapy alone encompassed 3435 patients, who were predominantly male, with a mean age of 57 to 63 years. The proportion of myocardial infarctions involving the anterior territory was 37% to 51%. Patients in cardiogenic shock were excluded or poorly represented in most trials. The fibrinolytic agent used was streptokinase in two trials (8,9), alteplase (rt-PA) in two trials (10,11) and tenecteplase (TNK) in three trials (12–14). In the ASSENT-4 and WEST studies (study acronyms are spelled out in Appendix 1), 20% and 40% of patients were enrolled in the prehospital setting, respectively. Patients were excluded if PCI could be performed less than 1 h after random assignment. In the LIMI and PRAGUE-1 studies, all patients were enrolled in non-PCI-capable hospitals and transferred to PCI-capable centres. Their maximum travel time was 60 min and their mean travel times were 20 min and 37 min, respectively. Both required transport to be available less than 1 h after random assignment.
Time from fibrinolytic therapy to PCI (needle-to-balloon time) varied substantially among trials and may account for the heterogeneous outcomes observed. Five trials performed facilitated PCI at the earliest possible time, achieving needle-to-balloon times of 31 min to 104 min. Two trials, GRACIA-2 and WEST, performed facilitated PCI in a slightly delayed fashion, achieving needle-to-balloon times of approximately 300 min (3 h to 12 h after fibrinolysis in the former and less than 24 h after random assignment in the latter). In the case of failed ST segment resolution or hemodynamic or electrical instability, rescue PCI was performed without delay. Nonfacilitated PCI was performed at the earliest possible time (less than 3 h after random assignment). Interestingly, ASSENT-4 also intended on performing facilitated PCI in a slightly delayed fashion (at least 90 min after random assignment), but the Food and Drug Administration believed that it would be unethical and mandated earlier PCI.
The impact of needle-to-balloon time is seen by comparing ASSENT-4 with GRACIA-2. These trials had similar patient populations and facilitating regimens (full-dose TNK), but dissimilar needle-to-balloon times and observed outcomes. TIMI-3 flow was lower in ASSENT-4 (43% versus 67%), corresponding to the shorter needle-to-balloon time (104 min versus 300 min). TIMI-3 flow was lowest in PACT (33%), corresponding to the shortest needle-to-balloon time (49 min). The risk of major bleeding was 5.6% to 10.0% in the five trials performing facilitated PCI at the earliest possible time compared with 1.9% to 2.0% in the two trials performing it after a slight delay. Thus, shorter needle-to-balloon time was associated with decreased TIMI-3 flow at initial angiography and increased major bleeding in patients receiving facilitated PCI.
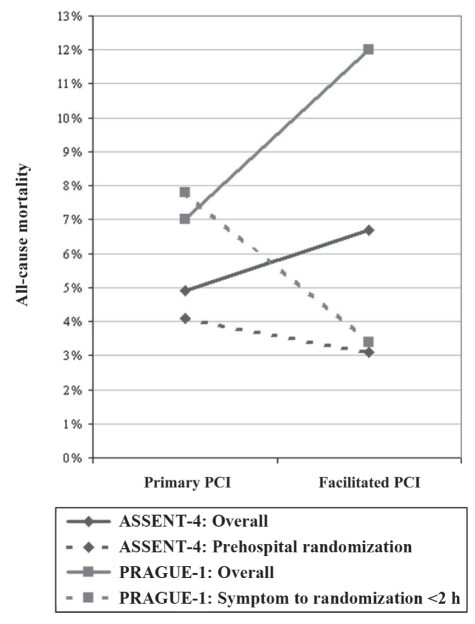
Conversely, shorter symptom onset-to-needle time was associated with a trend toward reduced mortality in patients receiving facilitated PCI. In PRAGUE-1, patients enrolled less than 2 h after symptom onset had a 4.4% reduction in cardiac mortality with facilitated PCI compared with nonfacilitated PCI (3.4% versus 7.8%; RR 0.44, 95% CI 0.08 to 2.30). In ASSENT-4, patients enrolled in the prehospital setting had a trend toward reduced mortality with facilitated PCI compared with nonfacilitated PCI (3.1% versus 4.1%; RR 0.74, 95% CI 0.24 to 2.30). The risk of mortality was lowest in WEST (1% versus 1%; RR 0.96, 95% CI 0.06 to 15.16), which had the largest proportion of patients enrolled and treated in the prehospital setting. The marked impact of symptom onset-to-needle time is shown in Figure 2.
Figure 2).
The impact of early fibrinolysis. PCI Percutaneous coronary intervention
These provocative results should not be interpreted definitively because these trials had small sample sizes and were underpowered to detect differences in mortality. The largest trial to-date, ASSENT-4, enrolled 1667 of a planned 4000 patients before being prematurely terminated because of increased in-hospital mortality with facilitated PCI. The increased in-hospital mortality was no longer significant when 90-day follow-up became available. At 90 days, there were 55 deaths with facilitated PCI and 41 deaths with primary PCI. Remarkably, 24 of the 55 facilitated PCI deaths were reported in two of the 24 participating countries. The details surrounding the two countries have not yet been elucidated.
Antiplatelet and antithrombin therapies varied within and among trials. In ASSENT-4 and GRACIA-2, facilitated PCI patients did not receive GP IIb/IIIa inhibitors (except in ‘bail-out’ situations), whereas nonfacilitated PCI patients received GP IIb/IIIa inhibitors at the discretion of the treating physicians. In WEST, facilitated PCI patients did not receive clopidogrel, whereas all nonfacilitated PCI patients received clopidogrel in the emergency room. Patients randomly assigned to facilitated PCI received half-dose unfractionated heparin in LIMI, no unfractionated heparin in PRAGUE-1 and a reduced bolus with no infusion in ASSENT-4. The conservative use of antiplatelet and antithrombin therapies may be responsible for the heightened risk of reinfarction with facilitated PCI.
The negative effects of facilitated PCI on intracranial hemorrhage and reinfarction were consistent across trials. The risk of intracranial hemorrhage was 0.0% to 2.7% with facilitated PCI and 0.0% to 0.3% with nonfacilitated PCI. This was statistically significant in ASSENT-4, and was the second leading cause of death (occurring in facilitated PCI patients only). The risk of reinfarction was 2.0% to 7.0% with facilitated PCI and 1.0% to 3.7% with non-facilitated PCI. In addition, facilitated PCI had no effect on the following surrogate end points: ST segment resolution at 60 min (GRACIA-2 [12], ADVANCE MI [15]), infarct size (GRACIA-2, BRAVE [16]), convalescent LVEF (SANI [8], PACT [10], PRAGUE-1 [17], GRACIA-2, BRAVE [16]), exercise stress test performance (PACT [10]) or MACE up to one year of follow-up (LIMI [11], PACT [10], PRAGUE-1 [18]).
In WEST, facilitated PCI did improve enzymatic infarct size measured by peak creatine kinase (1590 U versus 1833 U, P=0.045), N-terminal pro-B-type natriuretic peptide at 72 h (P=0.092) and QRS score on the discharge electrocardiogram. These data were strengthened by a trend toward reduced congestive heart failure at 30 days (14.4% versus 18.0%; RR 0.80, 95% CI 0.43 to 1.50) and reduced cardiogenic shock at 30 days (3.8% versus 7.0%; RR 0.55, 95% CI 0.17 to 1.82). In PACT, the potential for myocardial salvage with efficacious fibrinolysis was also seen. When TIMI-3 flow at initial angiography was successfully achieved, facilitated PCI improved convalescent LVEF by 4.5% (62.4% versus 57.9%, P=0.004).
PCI facilitated by half-dose fibrinolytic therapy plus GP IIb/IIIa inhibitors
By lysing fibrin, fibrinolysis exposes thrombin particles, which stimulate platelet activation and aggregation. Activated platelets release plasminogen activator inhibitor-1, which inhibits endogenous fibrinolysis (19). Moreover, platelet-rich clots are inherently resistant to fibrinolysis, and experimental models have shown that concomitant GP IIb/IIIa inhibition accelerates clot lysis threefold (20). Thus, GP IIb/IIIa inhibitors act synergistically to increase the efficacy of fibrinolysis and counteract its prothrombotic properties.
GUSTO V and ASSENT-3 trials compared half-dose fibrinolytic therapy plus abciximab with full-dose fibrinolytic therapy (without routine PCI) (21–24). Combination pharmacotherapy was associated with a 2% decrease in reinfarction, no difference in mortality up to one year and a 2% increase in major bleeding. These findings were consistent with a meta-analysis (25) of abciximab administered before reperfusion therapy. In the current era of primary PCI, two trials re-evaluated combination pharmacotherapy with routine PCI.
In BRAVE (16), 253 patients were randomly assigned to half-dose reteplase (r-PA) plus abciximab followed by angiography, or abciximab alone followed by angiography. Seventy-four per cent of patients were enrolled at non-PCI-capable hospitals and were transferred for PCI at a mean transport time of 35 min. TIMI-3 flow at initial angiography was greater in patients who received r-PA plus abciximab (40%) compared with patients who received abciximab alone (18%). When patients presenting 6 h to 12 h after symptom onset were excluded, TIMI-3 flow with half-dose r-PA plus abciximab was 50%. Scintigraphic infarct size, the primary outcome measure, was similar regardless of reperfusion strategy. Post hoc analyses suggested that the study may have been underpowered to detect true differences between groups.
ADVANCE MI (15) was similarly underpowered when study sponsors prematurely terminated the trial after 148 of a planned 5640 patients had been enrolled. The reason for the termination was insufficient enrolment. Patients were randomly assigned to half-dose TNK plus eptifibatide followed by angiography within 4 h, or placebo plus eptifibatide followed by angiography within 4 h. Combination pharmacotherapy was associated with a twofold increase in TIMI-3 flow at initial angiography, but no change in TIMI blush score, no change in mortality or reinfarction, and a twofold increase in major bleeding. Intention-to-treat and per-protocol analyses provided comparable conclusions.
The FINESSE trial (26) was published after the present systematic review had been completed. Like ADVANCE MI, study sponsors prematurely terminated the trial after 2452 of a planned 3000 patients were enrolled due to insufficient enrollment and cost overruns. Based on the observation that the benefits of faster reperfusion were exponentially greater among higher risk patients (27,28), the FINESSE trial targeted moderate-to-high risk patients, including those who were older than 75 years of age and excluding those who were younger than 60 years of age with localized inferior infarctions (29). Patients were randomly assigned to half-dose r-PA plus abciximab, abciximab alone or placebo, each followed by angiography within 1 h to 4 h and PCI if indicated. The results of this trial were recently reported, globally showing no advantage of facilitated PCI over nonfacilitated PCI for reducing the composite end point of all-cause mortality, cardiogenic shock, heart failure or ventricular fibrillation (9.8% versus 10.7%; HR 0.91, 95% CI 0.67 to 1.23) leading to the commentary that “it is time to abandon the routine use of combination-facilitated PCI” (30).
DISCUSSION
TIMI-3 flow at initial angiography and observed outcomes
Facilitated PCI did not improve outcomes despite a (less than expected) increase in TIMI-3 flow at initial angiography. TIMI-3 flow at initial angiography has been shown to be a strong predictor of mortality in trials of fibrinolytic therapy (4,31,32) and trials of primary PCI (33–35). However, improved TIMI-3 flow at initial angiography was not clinically correlated with improved outcomes in trials of facilitated PCI. We propose three potential explanations for the inconsistent relationship between TIMI-3 flow and observed outcomes.
First, faster IRA patency was counterbalanced by increased reinfarction (36). The combination of fibrinolysis followed by PCI creates a strongly prothrombotic environment, where reinfarction is more likely to occur. Fibrinolysis is associated with thrombin generation and platelet activation. PCI is associated with endothelial injury and implantation of foreign thrombogenic intracoronary stents. GP IIb/IIIa inhibitors (incompletely [37]) mitigate the prothrombotic effects of fibrinolytic therapy at the expense of increased major bleeding. Clopidogrel may be an attractive alternative to GP IIb/IIIa inhibitors because it has been shown to reduce reinfarction without increasing major bleeding in patients treated with fibrinolytic therapy and subsequent PCI (38).
Second, the proportion of patients who had TIMI-3 flow with fibrinolytic therapy was lower than the expected 66% previously shown in TIMI-10B (39). GRACIA-2 and BRAVE included patients presenting 6 h to 12 h after symptom onset who were less likely to reperfuse successfully with fibrinolytic therapy. PRAGUE-1 and SANI administered streptokinase, which is less effective than newer, fibrin-specific agents. Most trials performed PCI very soon after fibrinolytic therapy had been administered, not allowing sufficient time for reperfusion to occur. Inclusion of late-presenting patients, use of fibrin-nonspecific agents, and short time between fibrinolytic therapy and angiography may explain why TIMI-3 flow was generally sub-par.
Last, TIMI-3 flow was not correlated with microvascular reperfusion, as reflected by TIMI blush score and ST segment resolution. The importance of achieving both macrovascular and microvascular reperfusion was shown in the TIMI STEMI database of fibrinolysis (40). Patients who achieved the ‘trifecta of reperfusion’ (TIMI-3 flow, TIMI-3 blush, and 70% or greater ST segment resolution) had a 0.9% risk of death or congestive heart failure, whereas patients who achieved none to two of the aforementioned had a 9.2% to 12.4% risk. TIMI-3 flow alone is an ‘angiographic snapshot’ (41,42) that tends to overestimate the efficacy of fibrinolytic therapy on downstream microvascular reperfusion (43,44).
Timing of fibrinolysis and subsequent PCI
The clinical benefits of reperfusion therapy are greatest when initiated less than 2 h after symptom onset (45). During this time, myocardial injury is reversible and manifests as intracellular edema (46). When initiated 2 h to 12 h after symptom onset, myocardial injury is irreversible and manifests as coagulation necrosis, with the wavefront phenomenon of extension from central ischemic myocardium toward peripheral at-risk myocardium (47). In contrast with the early phase, mortality and myocardial salvage are influenced by time-independent (or ‘less dependent’) effects of reperfusion therapy, collateral circulation, ischemic preconditioning and myocardial oxygen demand (41,48). Moreover, reperfusion therapy may be associated with adverse effects such as contraction band necrosis (caused by rapid influx of calcium into dying cells), reperfusion injury, intramural hemorrhage, iatrogenic coronary artery dissection and distal microvascular embolization (49,50).
Administering fibrinolytic therapy less than 2 h after symptom onset, more readily accomplished in the prehospital setting, halts myocardial injury at the reversible stages. Subgroup analyses of pre-hospital fibrinolysis from ASSENT-4 and early presenting patients from PRAGUE-1, support the findings of the CAPTIM trial (51). In CAPTIM, 834 patients were randomly assigned to prehospital rt-PA and transfer versus direct transfer for primary PCI. Patients who received prehospital rt-PA less than 2 h after symptom onset had a 3.5% absolute reduction in mortality. Because fibrinolytic therapy was not routinely followed by angiography, CAPTIM was not a trial of facilitated PCI per se.
Performing PCI 3 h to 24 h after fibrinolytic therapy allows sufficient time for reperfusion to occur and for risks of bleeding complications to diminish. GRACIA-2 and WEST performed facilitated PCI in a slightly delayed fashion and had the lowest rates of mortality and bleeding complications. At the opposite end of the spectrum, ADVANCE MI performed facilitated PCI in a very rapid fashion, and the mortality was 6.8% with this approach compared with 0.0% with the nonfacilitated approach. In the context of facilitated PCI, achieving a rapid door-to-balloon time may not be advantageous if the patient is stable and there is no objective evidence of ongoing infarction.
The strategy of slightly delayed facilitated PCI used in GRACIA-2 and WEST may be particularly relevant in nonurban centres that are geographically removed from PCI facilities. Although immediate transfer for primary PCI has been shown to be superior to onsite fibrinolytic therapy in patients presenting to hospitals without PCI facilities, transfer delays never exceeded 3 h in these trials (52). Conversely, intrinsic transportation delays often exceed 3 h in many remote Canadian regions (and in other regions of the world), making the strategy of slightly delayed facilitated PCI a tenable option deserving of further study.
CONCLUSIONS
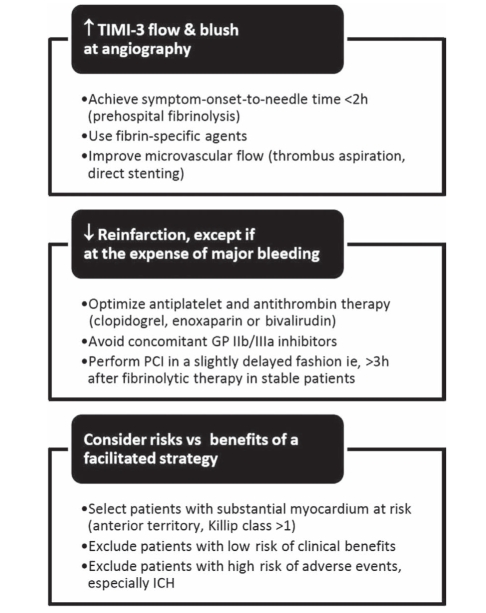
Our systematic review offers promising insights into the heterogeneous and complex trials of fibrinolytic-facilitated PCI, as summarized in Figure 3. This strategy may be beneficial if fibrinolytic therapy is initiated less than 2 h after symptom onset (mainly in the prehospital setting), and if PCI is slightly delayed 3 h to 24 h after fibrinolytic therapy in stable patients or remote regions. Overall, facilitated PCI does not improve survival, and increases intracranial hemorrhage and reinfarction compared with nonfacilitated PCI. Combining half-dose fibrinolytic therapy and GP IIb/IIIa inhibitors reduces reinfarction, but increases major bleeding. As such, facilitated PCI cannot be recommended at this time outside of experimental protocols. Further research should focus on selecting patients with higher benefit-to-risk ratios, performing earlier fibrinolysis and later PCI (when appropriate), and coupling reperfusion therapy with optimal antiplatelet and antithrombin therapy.
Figure 3).
Future challenges for facilitated percutaneous coronary intervention (PCI). GP Glycoprotein; ICH Intracranial hemorrhage; TIMI-3 Thrombolysis In Myocardial Infarction grade 3; vs Versus
TABLE 2.
Characteristics of trials using combination fibrinolytics and glycoprotein (GP) IIb/IIIa inhibitors for facilitated percutaneous coronary intervention (PCI)
| Study design | BRAVE (16) 2004 | ADVANCE MI (15) 2005 |
|---|---|---|
| Participants, n | 253 | 148 |
| Age, years (mean ± SD) | 63 (N/R) | 57 (N/R) |
| Female sex, % | 24 | 25 |
| Anterior territory, % | 42 | 30 |
| Fac PCI regimen | Half-dose r-PA + abciximab + PCI | Half-dose TNK + eptifibatide + PCI |
| Primary PCI regimen | Abciximab + PCI | Placebo + eptifibatide + PCI |
| Time delays | Fac | Nonfac | Fac | Nonfac |
|---|---|---|---|---|
| Symptom-to-randomization, min (median) | 130 | 140 | 150 | 132 |
| Randomization-to-drug, min (median) | 30 | 24 | N/R | N/R |
| Drug-to-angiography, min (median) | 125 | 120 | N/R | N/R |
| Randomization-to-angiography, min (median) | 155 | 144 | 84 | 84 |
| Concomitant therapies | Fac | Nonfac | Fac | Nonfac |
|---|---|---|---|---|
| Acetylsalicylic acid | All | All | All | All |
| Heparin | 60 U/kg | 60 U/kg | 40 U/kg + infusion or enoxaparin | 40 U/kg + infusion or enoxaparin |
| Intracoronary stents | 92% | 89% | ||
| Thienopyridines post-PCI | All | All | All stents | All stents |
| GP IIb/IIIa inhibitors post-PCI | All | All | All | All |
Fac Facilitated PCI; N/R Not reported; Nonfac Nonfacilitated PCI; r-PA Reteplase; TNK Tenecteplase
TABLE 3.
Results of trials using fibrinolytics alone for facilitated percutaneous coronary intervention (PCI)
|
SANI (8) 1992 |
LIMI (11) 1999 |
PACT (10) 1999 |
PRAGUE-1 (9) 2000 |
ASSENT-4 (14) 2006 |
GRACIA-2 (12) 2007 |
WEST (13) 2006 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fac | Nonfac | Fac | Nonfac | Fac | Nonfac | Fac | Nonfac | Fac | Nonfac | Fac | Nonfac | Fac | Nonfac | |
| Angiographic outcomes | ||||||||||||||
| TIMI-3 flow at initial angiography, % | 19* | 24* | 58† | 11† | 33† | 15† | 30† | 12† | 43† | 15† | 67† | 14† | N/R | N/R |
| TIMI-3 flow after PCI, % | 85 | 87 | 82 | 92 | 77 | 79 | 91 | 92 | 88 | 89 | 87 | 84 | 81 | 90 |
| TIMI blush score | N/R | N/R | N/R | N/R | N/R | N/R | N/R | N/R | N/R | N/R | 2–3: 55%† | 2–3: 17%† | N/R | N/R |
| Left ventricular ejection fraction, % | 51 | 51 | N/R | N/R | 58 | 58 | 50 | 51 | N/R | N/R | 59 | 56 | N/R | N/R |
| Clinical outcomes, % | ||||||||||||||
| Mortality‡ | 6.5 | 5.1 | 8.1§ | 6.7§ | 3.6 | 3.3 | 12.0§ | 7.0§ | 6.7¶ | 4.9¶ | 2.9 | 5.6 | 1.0 | 1.0 |
| Reinfarction | N/R | N/R | 5.4 | 1.3 | 3.0 | 2.6 | 7.0 | 1.0 | 6.1† | 3.7† | 1.0 | 0.9 | 5.8 | 3.0 |
| Reintervention | 10.3† | 1.6† | 23.0 | 8.0 | 7.3 | 7.2 | 7.0 | 6.9 | 6.6† | 3.4† | 6.7 | 6.5 | N/R | N/R |
| Congestive heart failure | N/R | N/R | N/R | N/R | N/R | N/R | 15.0 | 8.0 | 12.0 | 9.2 | N/R | N/R | 14.4 | 18.0 |
| Major adverse cardiac events** | N/R | N/R | 14.0 | 8.0 | N/R | N/R | 15.0 | 8.0 | 18.6† | 13.4† | 9.6 | 12.0 | 24.0 | 23.0 |
| Safety outcomes, % | ||||||||||||||
| Any stroke | N/R | N/R | 4.1 | 2.7 | 0.7 | 0.7 | 3.0 | 0 | 2.7† | 0.1† | 1.0 | 0.0 | 1.0 | 1.0 |
| Intracranial hemorrhage | 1.7 | 0 | 2.7 | 0 | 0.3 | 0.3 | 1.0 | 0 | 1.1† | 0.1† | 1.0 | 0 | 0 | 0 |
| Major bleeding episode | 10.0 | 0 | 0 | 0 | 8.5 | 8.2 | 7.2 | 0 | 5.6 | 4.4 | 1.9 | 2.8 | 1.9 | 1.0 |
| Transfusion requirement | 39.0† | 8.0† | N/R | N/R | N/R | N/R | N/R | N/R | 6.2 | 4.2 | N/R | N/R | N/R | N/R |
SANI Thrombolysis In Myocardial Infarction (TIMI) grade 2 or 3 flow at intial angiography performed before streptokinase administration;
Significant difference P<0.05;
Mortality – LIMI: 42-day; PACT: 30-day; PRAGUE-1: 30-day; GRACIA-2: 45-day; ASSENT-4: 90-day; WEST: 30-day;
1-year mortality – LIMI: facilitated PCI (Fac) 9.5% versus nonfacilitated PCI (Nonfac) 12.0%; PRAGUE-1: Fac 12.0% versus Nonfac 13.0%;
ASSENT-4 mortality by site of randomization – PCI hospital: Fac 8.5% versus Nonfac 5.2%; Non-PCI hospital: Fac 5.3% versus Nonfac 4.8%; Ambulance: Fac 3.1% versus Nonfac 4.1%;
Major adverse cardiac events – LIMI: 42-day death or reinfarction; PACT: 30-day death or reinfarction or stroke; GRACIA-2: 6-month death or reinfarction or repeat revascularization; ASSENT-4: 90-day death or congestive heart failure or cardiogenic shock; WEST: 30-day death or reinfarction or heart failure or cardiogenic shock or refractory ischemia or major ventricular arrhythmias. N/R Not reported
Acknowledgments
The abstract was presented in part at the World Congress of Cardiology, Barcelona, September 5, 2006.
APPENDIX 1
Study acronyms
| ASSENT-4: Assessment of the Safety and Efficacy of a New Thrombolytic Regimen – 4 |
| ADVANCE MI: Addressing the Value of Facilitated Angioplasty after Combination Therapy or Eptifibatide Monotherapy in Acute Myocardial Infarction |
| BRAVE: Bavarian Reperfusion Alternatives Evaluation |
| CAPTIM: Comparison of Angioplasty and Prehospital Thrombolysis in Acute Myocardial Infarction |
| CARESS in AMI: Combined Abciximab REteplase Stent Study in Acute Myocardial Infarction |
| FINESSE: Facilitated Intervention with Enhanced Reperfusion Speed to Stop Events |
| GRACIA-2: Grupo de Análisis de la Cardiopatía Isquémica Aguda – 2 |
| GUSTO V: Global utilization of Streptokinase and Tissue plasminogen activator for Occluded coronary arteries – V |
| LIMI: Prospective randomised comparison between thrombolysis, rescue PTCA, and primary PTCA in patients with extensive myocardial infarction admitted to a hospital without PTCA facilities: A safety and feasibility study |
| PACT: Plasminogen-activator Angioplasty Compatibility Trial |
| PRAGUE-1: PRimary Angioplasty in patients transferred from General community hospitals to specialized PTCA Units with or without Emergency thrombolysis – 1 |
| SANI A: Prospective, Placebo-Controlled, Randomized Trial of Intravenous Streptokinase and Angioplasty Versus Lone Angioplasty Therapy of Acute Myocardial Infarction |
| TRANSFER-AMI: Trial of Routine ANgioplasty and Stenting after Fibrinolysis to Enhance Reperfusion in Acute Myocardial Infarction |
| WEST: Which Early ST-elevation myocardial infarction Therapy |
REFERENCES
- 1.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: A quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 2.Keeley EC, Boura JA, Grines CL.Comparison of primary and facilitated percutaneous coronary interventions for ST-elevation myocardial infarction: Quantitative review of randomised trials Lancet 2006367579–88.(Erratum in 2006;367:1656). [DOI] [PubMed] [Google Scholar]
- 3.The Thrombolysis in Myocardial Infarction (TIMI) trial Phase I findings. TIMI Study Group. N Engl J Med. 1985;312:932–6. doi: 10.1056/NEJM198504043121437. [DOI] [PubMed] [Google Scholar]
- 4.Gibson CM, Cannon CP, Murphy SA, Marble SJ, Barron HV, Braunwald E, TIMI Study Group Relationship of the TIMI myocardial perfusion grades, flow grades, frame count, and percutaneous coronary intervention to long-term outcomes after thrombolytic administration in acute myocardial infarction. Circulation. 2002;105:1909–13. doi: 10.1161/01.cir.0000014683.52177.b5. [DOI] [PubMed] [Google Scholar]
- 5.Konkle BA, Schafer AI. Hemostasis, thrombosis, fibrinolysis, and cardiovascular disease. In: Zipes DP, Libby P, Bonow RO, Braunwald E, editors. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 7th edn. Philadelphia: Elsevier Saunders; 2005. pp. 2085–6. [Google Scholar]
- 6.Le May MR, Wells GA, Labinaz M, et al. Combined angioplasty and pharmacological intervention versus thrombolysis alone in acute myocardial infarction (CAPITAL AMI study) J Am Coll Cardiol. 2005;46:417–24. doi: 10.1016/j.jacc.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 7.Cantor WJ, Brunet F, Ziegler CP, Kiss A, Morrison LJ. Immediate angioplasty after thrombolysis: A systematic review. CMAJ. 2005;173:1473–81. doi: 10.1503/cmaj.045278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Neill WW, Weintraub R, Grines CL, et al. A prospective, placebo-controlled, randomized trial of intravenous streptokinase and angioplasty versus lone angioplasty therapy of acute myocardial infarction. Circulation. 1992;86:1710–7. doi: 10.1161/01.cir.86.6.1710. [DOI] [PubMed] [Google Scholar]
- 9.Widimský P, Groch L, Zelízko M, Aschermann M, Bednár F, Suryapranata H. Multicentre randomized trial comparing transport to primary angioplasty vs immediate thrombolysis vs combined strategy for patients with acute myocardial infarction presenting to a community hospital without a catheterization laboratory. The PRAGUE study. Eur Heart J. 2000;21:823–31. doi: 10.1053/euhj.1999.1993. [DOI] [PubMed] [Google Scholar]
- 10.Ross AM, Coyne KS, Reiner JS, et al. A randomized trial comparing primary angioplasty with a strategy of short-acting thrombolysis and immediate planned rescue angioplasty in acute myocardial infarction: The PACT trial. PACT investigators. Plasminogen-activator Angioplasty Compatibility Trial. J Am Coll Cardiol. 1999;34:1954–62. doi: 10.1016/s0735-1097(99)00444-1. [DOI] [PubMed] [Google Scholar]
- 11.Vermeer F, Oude Ophuis AJ, vd Berg EJ, et al. Prospective randomised comparison between thrombolysis, rescue PTCA, and primary PTCA in patients with extensive myocardial infarction admitted to a hospital without PTCA facilities: A safety and feasibility study. Heart. 1999;82:426–31. doi: 10.1136/hrt.82.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernández-Avilés F, Alonso JJ, Peña G, et al. GRACIA-2 (Groupo de Análisis de Cardiopatía Isquémica Aguda) Investigators Primary angioplasty vs. early routine post-fibrinolysis angioplasty for acute myocardial infarction with ST-segment elevation: The GRACIA-2 non-inferiority, randomized, controlled trial. Eur Heart J. 2007;28:949–60. doi: 10.1093/eurheartj/ehl461. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong PW, WEST Steering Committee A comparison of pharmacologic therapy with/without timely coronary intervention vs. primary percutaneous intervention early after ST-elevation myocardial infarction: The WEST (Which Early ST-elevation myocardial infarction Therapy) study. Eur Heart J. 2006;27:1530–8. doi: 10.1093/eurheartj/ehl088. [DOI] [PubMed] [Google Scholar]
- 14.Assessment of the Safety and Efficacy of a New Treatment Strategy with Percutaneous Coronary Intervention (ASSENT-4 PCI) investigators Primary versus tenecteplase-facilitated percutaneous coronary intervention in patients with ST-segment elevation acute myocardial infarction (ASSENT-4 PCI): Randomised trial. Lancet. 2006;367:569–78. doi: 10.1016/S0140-6736(06)68147-6. [DOI] [PubMed] [Google Scholar]
- 15.ADVANCE MI Investigators Facilitated percutaneous coronary intervention for acute ST-segment elevation myocardial infarction: Results from the prematurely terminated ADdressing the Value of facilitated ANgioplasty after Combination therapy or Eptifibatide monotherapy in acute Myocardial Infarction (ADVANCE MI) trial Am Heart J 2005150116–22.(Erratum in 2005;150:391). [DOI] [PubMed] [Google Scholar]
- 16.Kastrati A, Mehilli J, Schlotterbeck K, et al. Bavarian Reperfusion Alternatives Evaluation (BRAVE) Study Investigators Early administration of reteplase plus abciximab vs abciximab alone in patients with acute myocardial infarction referred for percutaneous coronary intervention: A randomized controlled trial. JAMA. 2004;291:947–54. doi: 10.1001/jama.291.8.947. [DOI] [PubMed] [Google Scholar]
- 17.Krupicka J, Widimský P, Nechvatál L, et al. Inter-hospital transport for primary angioplasty does not compromise left ventricular function: Six-month echocardiographic follow-up of the PRAGUE 1 Study. Jpn Heart J. 2003;44:313–22. doi: 10.1536/jhj.44.313. [DOI] [PubMed] [Google Scholar]
- 18.Bednár F, Widimský P, Krupicka J, Groch L, Aschermann M, Zelízko M, PRAGUE Study Group Investigators Interhospital transport for primary angioplasty improves the long-term outcome of acute myocardial infarction compared with immediate thrombolysis in the nearest hospital (one-year follow-up of the PRAGUE-1 study) Can J Cardiol. 2003;19:1133–7. [PubMed] [Google Scholar]
- 19.Hirashima O, Ogawa H, Oshima S, et al. Serial changes of plasma plasminogen activator inhibitor activity in acute myocardial infarction: Difference between thrombolytic therapy and direct coronary angioplasty. Am Heart J. 1995;130:933–9. doi: 10.1016/0002-8703(95)90191-4. [DOI] [PubMed] [Google Scholar]
- 20.Collet JP, Montalescot G, Lesty C, Weisel JW. A structural and dynamic investigation of the facilitating effect of glycoprotein IIb/IIIa inhibitors in dissolving platelet-rich clots. Circ Res. 2002;90:428–34. doi: 10.1161/hh0402.105095. [DOI] [PubMed] [Google Scholar]
- 21.Assessment of the Safety and Efficacy of a New Thrombolytic Regimen (ASSENT)-3 Investigators Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: The ASSENT-3 randomised trial in acute myocardial infarction. Lancet. 2001;358:605–13. doi: 10.1016/S0140-6736(01)05775-0. [DOI] [PubMed] [Google Scholar]
- 22.Sinnaeve PR, Alexander JH, Bogaerts K, et al. Efficacy of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: One-year follow-up results of the Assessment of the Safety of a New Thrombolytic-3 (ASSENT-3) randomized trial in acute myocardial infarction. Am Heart J. 2004;147:993–8. doi: 10.1016/j.ahj.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 23.Topol EJ; GUSTO V Investigators. Reperfusion therapy for acute myocardial infarction with fibrinolytic therapy or combination reduced fibrinolytic therapy and platelet glycoprotein IIb/IIIa inhibition: The GUSTO V randomised trial. Lancet. 2001;357:1905–14. doi: 10.1016/s0140-6736(00)05059-5. [DOI] [PubMed] [Google Scholar]
- 24.Lincoff AM, Califf RM, Van de Werf F, et al. Global Use of Strategies To Open Coronary Arteries Investigators (GUSTO) Mortality at 1 year with combination platelet glycoprotein IIb/IIIa inhibition and reduced-dose fibrinolytic therapy vs conventional fibrinolytic therapy for acute myocardial infarction: GUSTO V randomized trial. JAMA. 2002;288:2130–5. doi: 10.1001/jama.288.17.2130. [DOI] [PubMed] [Google Scholar]
- 25.De Luca G, Suryapranata H, Stone GW, et al. Abciximab as adjunctive therapy to reperfusion in acute ST-segment elevation myocardial infarction: A meta-analysis of randomized trials. JAMA. 2005;293:1759–65. doi: 10.1001/jama.293.14.1759. [DOI] [PubMed] [Google Scholar]
- 26.Ellis SG, Tendera M, de Belder MA, et al. FINESSE Investigators Facilitated PCI in patient with ST-elevation myocardial infarction. N Engl J Med. 2008;358:2205–17. doi: 10.1056/NEJMoa0706816. [DOI] [PubMed] [Google Scholar]
- 27.McNamara RL, Wang Y, Herrin J, et al. NRMI Investigators Effect of door-to-balloon time on mortality in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2006;47:2180–6. doi: 10.1016/j.jacc.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 28.Antoniucci D, Valenti R, Migliorini A, et al. Relation of time to treatment and mortality in patients with acute myocardial infarction undergoing primary coronary angioplasty. Am J Cardiol. 2002;89:1248–52. doi: 10.1016/s0002-9149(02)02320-2. [DOI] [PubMed] [Google Scholar]
- 29.Ellis SG, Armstrong P, Betriu A, et al. Facilitated Intervention with Enhanced Reperfusion Speed to Stop Events Investigators Facilitated percutaneous coronary intervention versus primary percutaneous coronary intervention: Design and rationale of the Facilitated Intervention with Enhanced Reperfusion Speed to Stop Events (FINESSE) trial. Am Heart J. 2004;147:E16. doi: 10.1016/j.ahj.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 30.Leopold JA. Does thrombolytic therapy facilitate or foil primary PCI? N Engl J Med. 2008;358:2277–9. doi: 10.1056/NEJMe0801533. [DOI] [PubMed] [Google Scholar]
- 31.Anderson JL, Karagounis LA, Califf RM. Metaanalysis of five reported studies on the relation of early coronary patency grades with mortality and outcomes after acute myocardial infarction. Am J Cardiol. 1996;78:1–8. doi: 10.1016/s0002-9149(96)00217-2. [DOI] [PubMed] [Google Scholar]
- 32.Lenderink T, Simoons ML, Van Es GA, Van de Werf F, Verstraete M, Arnold AE. Benefit of thrombolytic therapy is sustained throughout five years and is related to TIMI perfusion grade 3 but not grade 2 flow at discharge. The European Cooperative Study Group. Circulation. 1995;92:1110–6. doi: 10.1161/01.cir.92.5.1110. [DOI] [PubMed] [Google Scholar]
- 33.Stone GW, Cox D, Garcia E, et al. Normal flow (TIMI-3) before mechanical reperfusion therapy is an independent determinant of survival in acute myocardial infarction: Analysis from the primary angioplasty in myocardial infarction trials. Circulation. 2001;104:636–41. doi: 10.1161/hc3101.093701. [DOI] [PubMed] [Google Scholar]
- 34.De Luca G, Ernst N, Zijlstra F, et al. Preprocedural TIMI flow and mortality in patients with acute myocardial infarction treated by primary angioplasty. J Am Coll Cardiol. 2004;43:1363–7. doi: 10.1016/j.jacc.2003.11.042. [DOI] [PubMed] [Google Scholar]
- 35.Brodie BR, Stuckey TD, Hansen C, Muncy D. Benefit of coronary reperfusion before intervention on outcomes after primary angioplasty for acute myocardial infarction. Am J Cardiol. 2000;85:13–8. doi: 10.1016/s0002-9149(99)00598-6. [DOI] [PubMed] [Google Scholar]
- 36.Arnold AE, Serruys PW, Rutsch W, et al. Reasons for the lack of benefit of immediate angioplasty during recombinant tissue plasminogen activator therapy for acute myocardial infarction: A regional wall motion analysis. European Cooperative Study Group. European Cooperative Study Group. J Am Coll Cardiol. 1991;17:11–21. doi: 10.1016/0735-1097(91)90699-a. [DOI] [PubMed] [Google Scholar]
- 37.Mak KH, Lee LH, Wong A, et al. Thrombin generation and fibrinolytic activities among patients receiving reduced-dose alteplase plus abciximab or undergoing direct angioplasty plus abciximab for acute myocardial infarction. Am J Cardiol. 2002;89:930–6. doi: 10.1016/s0002-9149(02)02241-5. [DOI] [PubMed] [Google Scholar]
- 38.Sabatine MS, Cannon CP, Gibson CM, et al. Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY) – Thrombolysis in Myocardial Infarction (TIMI) 28 Investigators Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: The PCI-CLARITY study. JAMA. 2005;294:1224–32. doi: 10.1001/jama.294.10.1224. [DOI] [PubMed] [Google Scholar]
- 39.Cannon CP, Gibson CM, McCabe CH, et al. TNK-tissue plasminogen activator compared with front-loaded alteplase in acute myocardial infarction: Results of the TIMI 10B trial. Thrombolysis in Myocardial Infarction (TIMI) 10B Investigators. Circulation. 1998;98:2805–14. doi: 10.1161/01.cir.98.25.2805. [DOI] [PubMed] [Google Scholar]
- 40.Giugliano RP, Sabatine MS, Gibson CM, et al. Combined assessment of thrombolysis in myocardial infarction flow grade, myocardial perfusion grade, and ST-segment resolution to evaluate epicardial and myocardial reperfusion. Am J Cardiol. 2004;93:1362–6. doi: 10.1016/j.amjcard.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 41.Gersh BJ, Anderson JL. Thrombolysis and myocardial salvage. Results of clinical trials and the animal paradigm – paradoxic or predictable? Circulation. 1993;88:296–306. doi: 10.1161/01.cir.88.1.296. [DOI] [PubMed] [Google Scholar]
- 42.Lincoff AM, Topol EJ. Illusion of reperfusion. Does anyone achieve optimal reperfusion during acute myocardial infarction? Circulation. 1993;88:1361–74. doi: 10.1161/01.cir.88.3.1361. [DOI] [PubMed] [Google Scholar]
- 43.Steg PG, Thuaire C, Himbert D, et al. DECOPI Investigators DECOPI (DEsobstruction COronaire en Post-Infarctus): A randomized multi-centre trial of occluded artery angioplasty after acute myocardial infarction. Eur Heart J. 2004;25:2187–94. doi: 10.1016/j.ehj.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 44.Roe MT, Ohman EM, Maas AC, et al. Shifting the open-artery hypothesis downstream: The quest for optimal reperfusion. J Am Coll Cardiol. 2001;37:9–18. doi: 10.1016/s0735-1097(00)01101-3. [DOI] [PubMed] [Google Scholar]
- 45.Zijlstra F, Patel A, Jones M, et al. Clinical characteristics and outcome of patients with early (<2 h), intermediate (2–4 h) and late (>4 h) presentation treated by primary coronary angioplasty or thrombolytic therapy for acute myocardial infarction. Eur Heart J. 2002;23:550–7. doi: 10.1053/euhj.2001.2901. [DOI] [PubMed] [Google Scholar]
- 46.Vargas SO, Sampson BA, Schoen FJ. Pathologic detection of early myocardial infarction: A critical review of the evolution and usefulness of modern techniques. Mod Pathol. 1999;12:635–45. [PubMed] [Google Scholar]
- 47.Reimer KA, Jennings RB. The “wavefront phenomenon” of myocardial ischemic cell death. II. Transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral flow. Lab Invest. 1979;40:633–44. [PubMed] [Google Scholar]
- 48.Sadanandan S, Buller C, Menon V, et al. The late open artery hypothesis – a decade later. Am Heart J. 2001;142:411–21. doi: 10.1067/mhj.2001.117774. [DOI] [PubMed] [Google Scholar]
- 49.Kloner RA, Ellis SG, Lange R, Braunwald E. Studies of experimental coronary artery reperfusion. Effects on infarct size, myocardial function, biochemistry, ultrastructure and microvascular damage. Circulation. 1983;68:I8–15. [PubMed] [Google Scholar]
- 50.Waller BF, Rothbaum DA, Pinkerton CA, et al. Status of the myocardium and infarct-related coronary artery in 19 necropsy patients with acute recanalization using pharmacologic (streptokinase, r-tissue plasminogen activator), mechanical (percutaneous transluminal coronary angioplasty) or combined types of reperfusion therapy. J Am Coll Cardiol. 1987;9:785–801. doi: 10.1016/s0735-1097(87)80234-6. [DOI] [PubMed] [Google Scholar]
- 51.Steg PG, Bonnefoy E, Chabaud S, et al. Comparison of Angioplasty and Prehospital Thrombolysis In acute Myocardial infarction (CAPTIM) Investigators Impact of time to treatment on mortality after prehospital fibrinolysis or primary angioplasty: Data from the CAPTIM randomized clinical trial. Circulation. 2003;108:2851–6. doi: 10.1161/01.CIR.0000103122.10021.F2. [DOI] [PubMed] [Google Scholar]
- 52.Dalby M, Bouzamondo A, Lechat P, Montalescot G. Transfer for primary angioplasty versus immediate thrombolysis in acute myocardial infarction: A meta-analysis. Circulation. 2003;108:1809–14. doi: 10.1161/01.CIR.0000091088.63921.8C. [DOI] [PubMed] [Google Scholar]