Abstract
The wheat leaf-rust resistance gene Lr21 was first identified in an Iranian accession of goatgrass, Aegilops tauschii Coss., the D-genome donor of hexaploid bread wheat, and was introgressed into modern wheat cultivars by breeding. To elucidate the origin of the gene, we analyzed sequences of Lr21 and lr21 alleles from 24 wheat cultivars and 25 accessions of Ae. tauschii collected along the Caspian Sea in Iran and Azerbaijan. Three basic nonfunctional lr21 haplotypes, H1, H2, and H3, were identified. Lr21 was found to be a chimera of H1 and H2, which were found only in wheat. We attempted to reconstitute a functional Lr21 allele by crossing the cultivars Fielder (H1) and Wichita (H2). Rust inoculation of 5876 F2 progeny revealed a single resistant plant that proved to carry the H1H2 haplotype, a result attributed to intragenic recombination. These findings reflect how plants balance the penalty and the necessity of a resistance gene and suggest that plants can reuse “dead” alleles to generate new disease-resistance specificity, leading to a “death–recycle” model of plant-resistance gene evolution at simple loci. We suggest that selection pressure in crop–weed complexes contributes to this process.
PLANTS possess large numbers of resistance genes (R gene) as a part of an elaborate plant defense system. In different plants, an R-gene locus may consist of a single-copy (simple) or of multiple copies of R genes (complex) in clusters as a result of gene duplication events. This duplication is considered as the birth of an R gene. R genes are necessary for plants to respond to pathogen attacks and to survive when pathogens are in the environment. Mutations, gene conversion, and recombination were found to be the means to create new specificities for various pathogens (for review, Leister 2004). However, an R gene could bring a penalty when the pathogen is absent (Stahl et al. 1999). In such a case, plants have better fitness when they get rid of the R-gene function. In nature, the presence of different pathogens maintains the diversity of R-gene specificities. So far, there is no report on the fates of nonfunctional R genes.
In native agricultural ecosystems, wild plants often grow as weeds intermixed with or adjacent to their crop relatives. Extensive gene flow occurs between wild and domesticated forms, spawning numerous crop landraces adapted to diverse environments and occasionally new species. Common (hexaploid or bread) wheat (Triticum aestivum L., 2n = 6x = 42, genome formula AABBDD) arose from such a process by hybridization of domesticated tetraploid wheat (T. turgidum L., 2n = 4x = 28, AABB) with goatgrass (Aegilops tauschii Coss., 2n = 2x = 14, DD) growing as a weed in farmers' fields along the Caspian Sea in Iran ca. 8000 years ago (Kihara 1944; McFadden and Sears 1946; Nesbitt and Samuel 1998).
Because of the pivotal importance of Ae. tauschii in wheat evolution and crop improvement, Kihara et al. (1965) gathered extensive collections from Iran, Afghanistan, and adjacent regions. They suggested that Caspian Iran was the center of the genetic diversity of Ae. tauschii, a proposition later confirmed by molecular-marker analysis (Lubbers et al. 1991), as well as of resistance to leaf rust. Nine named and 12 new leaf-rust resistance genes have been documented in Ae. tauschii, and many more remain to be identified (Gill et al. 2008). Leaf rust, a scourge of wheat since before Roman times, is caused by the fungus Puccinia triticina (Eriks). It attacks mainly the leaf blade, producing small, elliptical, orange-red pustules on the upper surface, causing premature defoliation that results in as much as a 40% yield loss (McIntosh et al. 1995).
One Ae. tauschii accession, TA1599, collected in Caspian Iran, carries a gene named Lr21 that confers resistance to all known P. triticina races. Lr21, transferred to wheat in the 1970s (Rowland and Kerber 1974; McIntosh et al. 1995), was recently cloned (Huang et al. 2003) and shown to be a simple (single-copy) locus encoding a nucleotide-binding site–leucine-rich repeats (NBS–LRR) protein of 1080 amino acids. Here we report how a simple locus such as Lr21 evolved novel resistance specificities in a unique crop–weed system and how fragments of nonfunctional alleles could be reused in this process.
MATERIALS AND METHODS
Plant materials:
Twenty-five accessions of Ae. tauschii were used for this study (Table 1). Of these, 12 are the Lr21 carriers identified from the entire collection of 528 accessions of Ae. tauschii collected over a large geographic area representing its genetic diversity and maintained by the Wheat Genetic and Genomic Resources Center (WGGRC) at Manhattan, Kansas. Additional Lr genes are present in two accessions: Lr39 in TA2450 and Lr42 in TA2467. The remaining accessions carry no known Lr genes and are susceptible to leaf rust in the field. Among the 13 lr21 accessions, 5 were sampled at the same collection sites as the Lr21 accessions, 3 were collected along the Caspian Sea of Iran within 51 km of the Lr21 accessions, and the remaining 5 were from places where no Lr21 accessions were found.
TABLE 1.
Triticum and Aegilops accessions used for sequencing of Lr21 and lr21 alleles
| Accession | Species | Known Lr genes | Collection site | Polymorphism patterna |
|---|---|---|---|---|
| TA1599 | Ae. tauschii spp. tauschii | Lr21 | Ramsar, Iran | 1 |
| TA2527 | Ae. tauschii spp. tauschii | Lr21 | Ramsar, Iran | 2 |
| TA2528 | Ae. tauschii spp. tauschii | Lr21 | Ramsar, Iran | 2 |
| TA2529 | Ae. tauschii spp. tauschii | Lr21 | Ramsar, Iran | 4 |
| TA2530 | Ae. tauschii spp. tauschii | Lr21 | Ramsar, Iran | 3 |
| TA2472 | Ae. tauschii spp. tauschii | Lr21 | Ramsar, Iran | 2 |
| TA2378 | Ae. tauschii spp. tauschii | Lr21 | 9 km from Ramsar, Iran | 1 |
| TA2476 | Ae. tauschii spp. tauschii | 13 km from Ramsar, Iran | 9 | |
| TA2473 | Ae. tauschii spp. tauschii | 23 km from Ramsar, Iran | 9 | |
| TA1649 | Ae. tauschii spp. tauschii | Lr21 | Bandar-e Anzali, Iran | 1 |
| TA2477 | Ae. tauschii spp. tauschii | Bandar-e Anzali, Iran | 6 | |
| TA2481 | Ae. tauschii spp. tauschii | 12 km from Bandar-e Anzali, Iran | 7 | |
| TA2468 | Ae. tauschii spp. strangulata | Lr21 | 51 km North of Babolsar, Iran | 1 |
| TA2469 | Ae. tauschii spp. tauschii | 51 km North of Babolsar, Iran | 10 | |
| TA2470 | Ae. tauschii spp. strangulata | Lr21 | 51 km North of Babolsar, Iran | 3 |
| TA2471 | Ae. tauschii spp. strangulata | 51 km North of Babolsar, Iran | 7 | |
| TA2467 | Ae. tauschii spp. tauschii | Lr42 | 51 km North of Babolsar, Iran | 7 |
| TA2450 | Ae. tauschii spp. strangulata | Lr39 | 51 km North of Babolsar, Iran | 6 |
| TA1670 | Ae. tauschii spp. tauschii | Lr21 | Kutkashen, Azerbaijan | 1 |
| TA10110 | Ae. tauschii spp. tauschii | East of Chrelet Kopetdag mountain range, Turkmenistan | 5 | |
| TA2496 | Ae. tauschii spp. tauschii | Tabriz, Iran | 10 | |
| TA2495 | Ae. tauschii spp. tauschii | Tabriz, Iran | 10 | |
| TA1698 | Ae. tauschii spp. tauschii | Dagestan, Russia | 7 | |
| TA1699 | Ae. tauschii spp. tauschii | Dagestan, Russia | 7 | |
| TA1691 | Ae. tauschii spp. tauschii | Lr21 | Unknown | 3 |
| TA3009 | T. aestivum cv. Wichita | 9 | ||
| TA3908 | T. aestivum cv. Fielder | 9 |
Polymorphism patterns are based on KSUD14-STS marker analysis (Figure 2, A and B).
Of 24 wheat cultivars (Table 1 and Table S1) with the lr21 allele that were tested for polymorphism using the KSUD14-STS marker, a PCR-based molecular marker that distinguishes Lr21 from lr21 (Huang et al. 2003), “Fielder,” a spring wheat, and “Wichita,” a winter wheat, were chosen for this study. WGRC7 is a wheat germplasm developed by WGGRC (http://www.k-state.edu/wgrc/) by direct crossing Wichita with Ae. tauschii accession TA1649 and then backcrossing with Wichita twice (Raupp et al. 1983).
One dicot species, Arabidopsis thaliana, and five cereal species, barley (Hordeum vulgare), oat (Avena sativa), rye (Secale cereale), maize (Zea mays), and rice (Oryza sativa) were chosen on the basis of the evolutionary time line to assess the approximate age of the Lr21 locus.
DNA manipulation and sequence analysis:
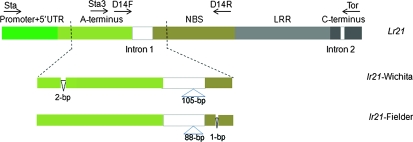
DNA isolation, digestion, blotting, and Southern hybridization followed the protocols described by Qi et al. (2004). Several pairs of primers were designed on the basis of coding regions and flanking sequences of Lr21 from Ae. tauschii accession TA1649. Primers Sta (TTGTGATGGAGAAACGAGTGGCC) and Tor (CGGACGAGTAGTTCTTTCAGGA) were designed to amplify the entire gene and 397-bp flanking regions (Figure 1). Each full-length allele was amplified by long-range PCR using Herculase-enhanced DNA polymerase (Stratagene, La Jolla, CA) from genomic DNA of each accession. The PCR products were then cloned directly using the pGEM-T easy system (Promega, Madison, WI). First, vector primers SP6 and T7 were used to sequence the ends of each clone. Internal primers were designed later on the basis of the sequences obtained from previous primers. At least three clones from each accession were sequenced from both directions. All the sequences were assembled using MacVector 6.5.3 (Oxford Molecular, Madison, WI). Primers Sta3 (TGGCTAATGCAGTGGGCACGG) and D14-R (GGACATTAGGCGATGCTTTGAATTC) were used to amplify the NBS region of the alleles (Figure 1). The marker KSUD14-STS was designed on the basis of a 105- or 88-bp insertion/deletion (indel) in the first intron of the Lr21 (Figure 1). A 1.36-kb fragment from this region is a signature of the Lr21, while a 1465-or 1448-bp fragment with a 105- or 88-bp insertion is a tag of lr21. Other sizes of fragments amplified with KSUD14-STS represent Lr21 paralogs (Figure 2, A and B).
Figure 1.—
Gene structure and primer location of the Lr21 and lr21 alleles. The enlarged regions show the major differences between the lr21-Wichita and the lr21-Fielder. A-terminus, amino terminus; NBS, nucleotide-binding site; LRR, leucine-rich repeats; and C terminus, carboxy terminus; 5′-UTR, 5′-untranslated region.
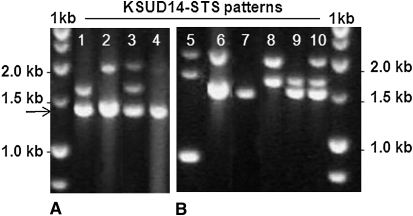
Figure 2.—
Polymorphism survey of Ae. tauschii based on the KSUD14-STS marker. (A) Four patterns (1–4) were revealed among 12 Lr21 accessions. The 1.36-kb fragment (indicated by an arrow) is a tag of Lr21, and other size fragments are Lr21 paralogs. (B) Six patterns (5–10) were identified among the 13 lr21 accessions, none of which has the 1.36-kb fragment.
Gene expression study:
Expression of the Lr21 and lr21 alleles was characterized by modified quantitative RT–PCR (Kashkush et al. 2003). The mRNA was isolated from leaf tissues with or without inoculation of the pathogen isolate PRTUS6 using MicroPoly(A) Pure (Ambion, Austin, TX). First-strand cDNAs were synthesized using oligo(dT) primer and second-strand cDNAs were amplified with gene-specific primers. Lr21 or lr21 were amplified with D14-F (CGAGATTGGTCCTATGAGGTGGT) and D14-R (Figure 1). Actin gene expression was used for normalization for the expression study. Actin-F (GGTATCGTGAGCAACTGGGATG) and Actin-R (GTGAAGGAGTAACCTCTCTCGGTG) were used to amplify a 383-bp fragment. PCR was performed under the following conditions: 95° for 4 min and 12 cycles each with 95° for 30 sec, 60° for 30 sec, and 72° for 1.5 min. The amplicons were separated by electrophoreses on 1% agarose gels, transferred to Hybond-N+ membranes (Amersham Biosciences, Piscataway, NJ), and probed with KSUD14 or the actin gene.
RESULTS
The Lr21 NBS–LRR family in cereal species:
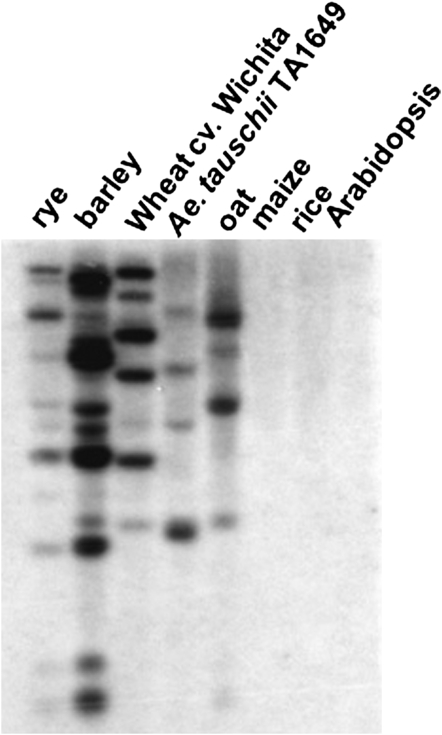
Hybridization of the Lr21 NBS region to wheat, rye, barley, and oat revealed multiple bands (Figure 3), indicating that lr21 homologs are present elsewhere in the genome and that the Lr21 NBS–LRR family is shared by this group of grasses with a basic chromosome number of 7. No signal was detected in maize, rice, and Arabidopsis (Figure 3), indicating that Lr21 homologs are absent in these species. Primers designed on the basis of the Lr21 NBS region were able to amplify fragments from rye, barley, and oat by PCR. Sequencing of the amplicons showed 95–98% similarity to the Lr21 NBS sequence (File S1). BLASTn searches against the finished rice genome sequence revealed eight rice homologs with E-values > −20 and identity from 57 to 70%, consistent with the failure of hybridization. None of the homologs were detected on rice chromosome 5, which is homeologous to the wheat group 1 chromosomes including 1D, where the Lr21 locus resides. Southern hybridization with probe KSUD14, a part of the Lr21 gene, revealed two Lr21 paralogs located on the short arm of chromosome 1D in the Lr21 donor accession of Ae. tauschii (Huang et al. 2003). Two cosmid clones carrying the paralogs were identified. Full-length sequencing of the paralogs showed them to be NBS–LRR-like sequences with 80% identity to Lr21 in the NBS region (File S2) and only ∼50% identity in the rest of the gene.
Figure 3.—
Southern hybridization of genomic DNAs digested with restriction enzyme XbaI and probed with KSUD14 (NBS region of Lr21). The hybridization stringency amounted to 80% homology.
Only one Lr21 allele was identified:
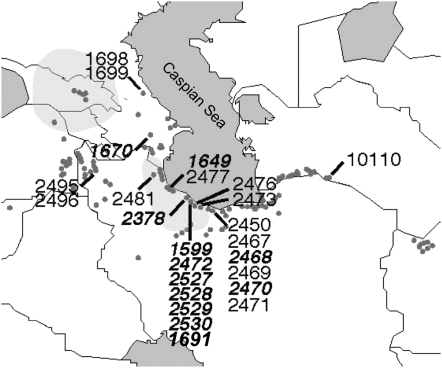
Of 528 accessions representing the geographic diversity of Ae. tauschii that were screened against a mixture of leaf-rust pathogens, 158 were found to be resistant at the seedling stage. The resistant accessions were tested with the Lr21-specific marker KSUD14-STS, and 12 were found to carry the Lr21 allele. All 12 had been collected along the Caspian Sea in Iran and Azerbaijan within a range of 675 km (Figure 4 and Table 1). They shared an identical 1.36-kb fragment at the Lr21 locus but were polymorphic at paralogous loci, with four patterns revealed by marker KSUD14-STS (Figure 2A). Amplification, cloning, and alignment of the Lr21 sequences showed that all 12 accessions carry an identical allele at the Lr21 locus.
Figure 4.—
The sampling sites and geographic distribution of the 25 Ae. tauschii accessions used in this study. Twelve accessions (in boldface italic type) are the Lr21 carriers.
The lr21 alleles are pseudogenes:
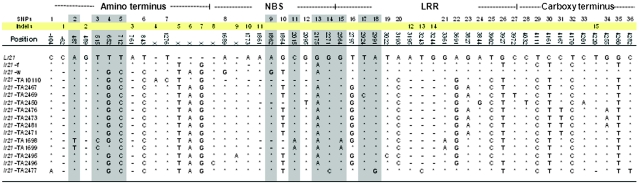
To elucidate the mechanisms underlying the origin of Lr21 function, we characterized nonfunctional lr21 alleles from 13 Ae. tauschii accessions (Figure 4 and Table 1) and two Ae. tauschii-derived lr21 alleles present in the D genome of two hexaploid wheat cultivars (Table 1). On the basis of KSUD14-STS genotyping in Ae. tauschii, six different polymorphic patterns were observed, but none contained the 1.36-kb fragment (Figure 2B). On the basis of sequencing, 11 different lr21 alleles were identified from the 13 Ae. tauschii accessions, of which TA2467, TA2473, and TA2481 carried the same allele (Figure 5, and Figure S1). The wheat D-genome alleles lr21-f (Fielder) and lr21-w (Wichita) were not detected in the sampled Ae. tauschii accessions. The sequences of the 11 lr21 Ae. tauschii and two wheat D-genome lr21 alleles revealed a spectrum of the accumulated variation at this locus (Figure 5). There were 36 single-nucleotide polymorphisms (SNPs), none introducing a stop codon and only 11 representing nonsynonymous substitutions. There were also 15 indels, 3 of which caused frameshifts that introduced premature stop codons. With reference to the Lr21 sequence, indel 3 (a 2-bp deletion at position +761) was present in all lr21 alleles except lr21-f and lr21-TA1699. This deletion introduced an early stop codon, which resulted in a putative 151-aa peptide. Similarly, indel 10, a 1-bp deletion at position +1773, was identified only in the lr21-f allele, which encodes a putative 380-aa peptide. Indel 12 was a 4-bp deletion at +3195 identified in 12 lr21 alleles, including the lr21-TA1699 allele. Each lr21 allele carried one or two of these three indels and was thus a nonfunctional pseudogene.
Figure 5.—
Sequence variation among tested alleles of Lr21 and lr21. Position numbers are based on the Lr21 sequence and are shown as offsets from the transcription start site. lr21-f is the Fielder allele and lr21-w is the Wichita allele. SNP, single-nucleotide polymorphism; indel, insertion/deletion. An asterisk indicates that the sequence is identical to Lr21, and a hyphen notes a deletion. Shaded positions are those in which the nucleotide change resulted in an amino-acid change. The X's represent deletions in Lr21. Four functional domains are indicated above the sequence: the amino terminus, NBS (nucleotide-binding site), LRR (leucine-rich repeat), and the carboxy terminus.
The two distinct lr21 alleles found in the wheat D genome, lr21-w and lr21-f, were identified from chromosome 1D of Wichita and Fielder, respectively (Figure 1). The lr21-w and lr21-f alleles were distributed among the other 22 sampled wheat cultivars (Table S1) without bias as to winter or spring habit. Both encoded truncated proteins, resulting from a 2-bp indel at position +761 in lr21-w and a 1-bp indel at +1773 in lr21-f (Figure 5), and thus were pseudogenes.
The Lr21 allele is a recombined allele of recent origin:
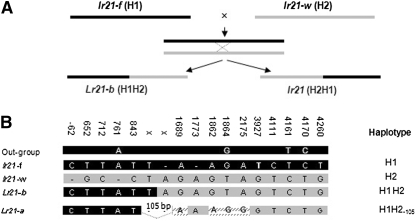
Sequences from the NBS and part of the 3′ regions of the gene from rye, barley, and oat revealed that the 2-bp insertion at indel 3 (position +761) (Figure 6B) and nucleotides G at position +1864, T at position +4161, and C at position +4170 are shared among the three outgroup species. The presence in the lr21-f allele of this 2-bp insertion at indel 3 and the ancestral SNPs (Figure 6B) support its ancestral character. Sequence comparisons suggested that the 13 different lr21 alleles were derived from three basic haplotypes and were subsequently modified by point mutation and insertion/deletion events (Figure 5 and Figure S1). We designated allele lr21-f as haplotype H1, lr21-w as haplotype H2, and lr21-TA2467 as haplotype H3. The Lr21 allele appears to be a chimera derived from intragenic recombination between H1 and H2 (Figure 5 and Figure S1), followed by the deletion of a 105-bp segment within the first intron, and was designated H1H2−105. The putative crossover site lies between positions +762 and +1772. The recombined allele could encode a full 1080-aa protein and would be free of both the 2-bp (in H2) and the 1-bp (in H1) deletions. Another chimeric haplotype, H2H1, appeared in the lr21-TA2477 allele, a putative product of reciprocal recombination carrying the 2-bp deletion (Figure 5 and Figure S1), and the lr21 protein product of this allele is truncated. The remaining lr21 alleles are suggested to have been derived from the H3 haplotype as shown in the supplemental data (Figure S1). All of the lr21 alleles are present as truncated pseudogenes but are transcribed as revealed by RT–PCR (Figure S2), suggesting that their promoters are still functioning.
Figure 6.—
(A) A crossing scheme for reconstituting a functional Lr21 allele from two nonfunctional alleles. (B) The indel and SNPs that are shared among the three outgroup species rye, barley, and oat and the region in which intragenic recombination between H1 and H2 resulted in the chimerical allele Lr21-b. Lr21-a is the allele identified from Ae. tauschii. Position numbers are based on the Lr21 sequence. In A and B, solid areas correspond to haplotype H1, representing allele lr21-f from cultivar Fielder, and shaded areas correspond to H2, representing lr21-w from Wichita. Hatching indicates SNPs that distinguish Lr21-b from Lr21-a.
A functional allele can be created from two dead alleles:
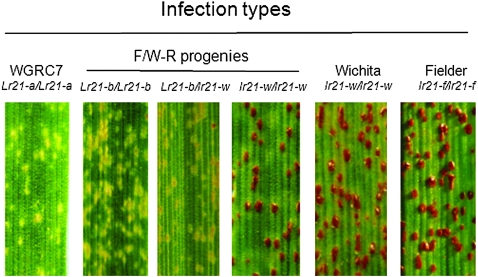
To test the hypothesis that Lr21 could originate from two dead (nonfunctional) alleles, we crossed wheat cultivars Wichita (H2) and Fielder (H1) (Figure 6A) and screened 5876 F2 progeny in the greenhouse using the leaf-rust isolate PRTUS6. One plant, a putative recombinant designated as F/W-R, was identified on the basis of having an infection type lower than that of both parents. The critical region between +762 and +1772 was PCR amplified from the F/W-R plant, cloned, and sequenced. Six of nine clones had sequences identical to H2. The other three had sequences identical to H1 from the 5′-end to position +843 and thereafter were identical to H2 (Figure 6B). These data indicated that F/W-R was heterozygous at the Lr21 locus for H2 and H1H2 haplotypes. The recombinant H1H2 was identical to the Lr21 of the Ae. tauschii gene (Lr21-a in Figure 6B) except for a 105-bp insertion derived from H2 and four substitutions: G to A at positions +1689 and +1862, T to G at position +1864, and A to G at position +2175 (Figure 5 and Figure 6B). The presence of this insertion and the four SNPs showed that the reconstituted Lr21-b functional allele was created in this cross. The 105-bp sequence lies in the first intron of the gene, and the deletion or insertion of this fragment does not change the length of the peptide encoded by the gene. Two of four substitutions are synonymous, while the one at position +1862 changes a methionine to a valine (both neutral and nonpolar), and the second at +2175 changes the acidic polar aspartic acid to the neutral nonpolar glycine. However, the reconstituted allele Lr21-b still confers resistance to the same pathogen isolate, indicating that the amino-acid changes at these positions do not change the function of the protein. The F/W-R plant was selfed. Fifty progenies were tested for a reaction to leaf-rust isolate PRTUS6. Progeny testing revealed that resistance to the leaf-rust isolate was conferred by a single dominant gene (Figure 7). Ten resistance and 10 susceptible plants were selected for genotyping using the critical region of the Lr21-b. The result suggested that the resistance was conferred by the Lr21-b allele.
Figure 7.—
Infection types of WGRC7 (Lr21-a/Lr21-a), progenies of the F/W recombinant (Lr21-b/Lr21-b ; Lr21-b/lr21-w; lr21-w/lr21-w), Fielder (lr21-f/lr21-f ) and Wichita (lr21-w/lr21-w) 9 days after inoculation with the leaf-rust isolate PRTUS6.
DISCUSSION
We selected the Lr21 gene for map-based cloning >10 years ago because the gene was identified in a dozen Ae. tauschii accessions spread over a large area in Caspian Iran. Our hypothesis was that Lr21 was probably a complex locus and was spawning new specificities as a result of unequal crossing over similar to the Rp1 locus of maize (Hulbert et al. 2001). It was surprising when molecular cloning revealed Lr21 to be a simple, single-copy locus (Huang et al. 2003). After investigating the sequence variation at the Lr21 locus in the 12 Lr21-carrier tauschii accessions and the 15 lr21 alleles in a sample of tauschii and bread wheat accessions, our results showed an unexpected monomorphism at the Lr21 locus among the 12 Lr21-carriers. The sequence data revealed Lr21 to be a chimeric allele, providing possible clues to its recent origin through intragenic recombination. This hypothesis was experimentally verified by the recovery of a functional allele in the progeny of a cross between two susceptible parents. These results have important implications about the age of the Lr21 locus, its origin, evolution, and fixation in the context of the crop–weed coevolutionary process as distinct from resistance evolution in wild populations; these aspects of this study are discussed below.
An ancient locus with a young allele of Lr21 NBS–LRR:
The evolutionary time line indicates that wheat diverged from rice and maize ∼65 MYA, from barley ∼14 MYA, and from rye ∼7 MYA (Huang et al. 2002). Compared to 80% identity with its paralogs (File S2), Lr21 shared >95% identity with barley, oat, and rye homologs in the NBS region (File S1). Since, in general, orthologous genes in different species are more similar in sequence to one another than paralogous copies within a species (Michelmore and Myers 1998; Hulbert et al. 2001), it is plausible that Lr21 is an ancient locus shared by wheat, barley, and oat. However, its restricted geographic distribution and DNA-level monomorphism strongly suggest that the Lr21 allele of Ae. tauschii originated more recently in a single event. In this scenario, it most likely spread by rare cross-pollination among Ae. tauschii populations, farming activity, and commerce of wheat grains contaminated with goatgrass seeds.
Chimeric origin of Lr21:
Ae. tauschii is a self-pollinated species with an outcrossing rate of <5%. The presence in the wheat D genome of the H1 and H2 haplotypes indicates that these alleles were present in Ae. tauschii (donor of the wheat D genome), growing alongside domesticated tetraploid wheat. It thus appears that Ae. tauschii parents carrying H1 and H2 or similar haplotypes were involved in hybridization events leading to the origin of bread wheat ca. 8000 years ago and that bread wheat originated in at least two independent hybridization events (Talbert et al. 1998). As H1 and H2 haplotypes were detected only in bread wheat, our failure to detect Ae. tauschii accessions carrying haplotypes H1 and H2 most probably was due to limited sampling. Alternatively, these haplotypes may be extinct in Ae. tauschii and preserved only in wheat.
The molecular mechanism underlying the origin of Lr21 function may be associated with its location at the most distal point of the chromosome 1D short arm, a recombination hot spot (Spielmeyer et al. 2000; Qi et al. 2004). We previously reported one intragenic recombination event between positions −61 and +1354 involving alleles Lr21 and lr21-w in a sample of 332 F2 plants (Huang et al. 2003). It involved a conversion tract of a minimum of 191 bp and a maximum of 1415 bp of DNA from lr21-w to Lr21, rendering the latter ineffective. We have now experimentally reconstituted Lr21 through another intragenic recombination event. In addition, one H2H1 haplotype, an obvious product of intragenic recombination, was detected in a small sample of 13 lr21 alleles. These findings suggest that recombination events have occurred multiple times at the Lr21 locus and that the evolutionary history of Lr21 has been shaped by its location in a high-recombination region.
“Birth–recycle” at the Lr21 locus:
The presence of only one functional allele among an assortment of nonfunctional alleles at the Lr21 locus suggests the cost of carrying the resistance allele in the absence of virulent pathogen strains. Two evolutionary classes of NBS–LRR genes have been characterized. One supports the so-called “arms race” model represented by the L locus of flax (Ellis et al. 1999) and the RPP13 locus of Arabidopsis (Rose et al. 2004), which contain large numbers of different functional alleles and a high degree of variation in the regions responsible for specificity. The other class, consistent with a “trench warfare” model, is represented by the RPM1 (Stahl et al. 1999) and RPS2 (Mauricio et al. 2003) loci of Arabidopsis in which variation is low with no evidence of diversifying selection between functional and nonfunctional forms. The functional RPM1 allele has been shown to impose a penalty in the absence of the pathogen. Complete deletion is one way to remove the deleterious effect of an allele such as RPM1. An alternative way is truncation, as seen with the lr21 alleles.
Our discovery suggests a “death–recycle” model of plant-resistance gene evolution at simple loci. A “birth-and-death” process similar to that of the vertebrate major histocompatibility complex, T-cell receptor, and immunoglobulin genes has been proposed to explain the evolution of resistance genes at complex loci (Michelmore and Myers 1998). At a simple locus, there is no “birth” associated with gene duplication. A functional allele may become ineffective because of mutation or defeat by a new race of the pathogen and then may be reused in the creation of a new functional allele at that locus. Our results have confirmed that plants can reuse nonfunctional alleles to create new resistance specificity. The recycling of lr21 hints at the potential usefulness of truncated alleles. New resistances similar to Lr21 that occurred in nature may also arise in plant breeding programs more often than recognized because of extensive selection pressure for rare new disease specificities in segregating populations subjected to disease epidemics.
The wheat–goatgrass complex and the fixation of Lr21:
Ae. tauschii accessions carrying the functional Lr21 allele are predominant in regions where agriculture was practiced, to the point where at one location, Ramsar, Iran, all collected accessions carried this allele (Figure 4 and Table 1). This predominance would be expected if some evolutinary process in the crop–weed agroecosystem led to fixation of a new disease-resistance specificity created by a rare recombination event in lr21. Agricultural practice favors crop monoculture, or a single variety planted over large areas for long periods of time. This often leads to much higher rust-disease pressure in wheat fields than in wild Ae. tauschii populations, which harbor mixtures of different rust-resistance genes or even several leaf-rust resistance genes in a single accession (Gill et al. 2008). We propose that leaf-rust epidemics in a crop monoculture imposed selection pressure on goatgrass populations in or near wheat fields. A plant carrying the Lr21 allele would have a fitness advantage in an environment with high leaf-rust inoculum. In this model, the crop–weed complex coevolutinary process was critical to the selection and retention of the Lr21 gene in Ae. tauschii populations.
It appears that native agricultural ecosystems, located in Vavilovian world centers of crop plant origin, are virtual outdoor laboratories for the creation of genetic variation. Other “new” genes spawned by such ecosystems may well be of the same worldwide economic significance as Lr21. The example presented here argues for the careful preservation of the native agricultural ecosystems in the face of modern agricultural practices because the success of modern plant breeding hinges on the extensive use of genetic variation present in land races and in wild relatives of crop plants.
Acknowledgments
We are grateful to Scot Hulbert, Frank White, and Eduard Akhunov for critical review of the manuscript. This research was supported by the Kansas Wheat Commission and a U. S. Department of Agriculture–Cooperative State Research, Education, and Extension Service special grant to the Wheat Genetic and Genomic Resources Center. This is contribution 07-305-J from the Kansas Agricultural Experiment Station, Manhattan, Kansas.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.108.099614/DC1.
References
- Ellis, J. G., G. J. Lawrence, J. E. Luck and P. N. Dodds, 1999. Identification of regions in alleles of the flax rust resistance gene L that determine differences in gene-for-gene specificity. Plant Cell 11 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill, B. S., L. Huang, V. Kuraparthy, W. J. Raupp, D. L. Wilson et al., 2008. Alien genetic resources for wheat leaf rust resistance, cytogenetic transfer, and molecular analysis. Aust. J. Agric. Res. 59 197–205. [Google Scholar]
- Huang, S., A. Sirikhachornkit, X. Su, J. Faris, B. S. Gill et al., 2002. Genes encoding plastid acetyl-CoA carboxylase and 3-phosphoglycerate kinase of the Triticum/Aegilops complex and the evolutionary history of polyploid wheat. Proc. Natl. Acad. Sci. USA 99 8133–8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, L., S. A. Brooks, W. Li, J. P. Fellers, H. N. Trick et al., 2003. Map-based cloning of leaf rust resistance gene Lr21 from the large and polyploid genome of bread wheat. Genetics 164 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert, S. H., C. A. Webb, S. M. Smith and Q. Sun, 2001. Resistance gene complexes: evolution and utilization. Annu. Rev. Phytopathol. 39 285–312. [DOI] [PubMed] [Google Scholar]
- Kashkush, K., M. Feldman and A. A. Levy, 2003. Transcriptional activation of retrotransposons alters the expression of adjacent genes in wheat. Nat. Genet. 33 102–106. [DOI] [PubMed] [Google Scholar]
- Kihara, H., 1944. Discovery of the DD-analyser, one of the ancestors of Triticum vulgare. Agric. Hortic. 19 13–14 (in Japanese). [Google Scholar]
- Kihara, H., K. Yamashita, and M. Tanaka, 1965. Results of the Kyoto University Scientific Expedition to the Karakoram and Hindukush. Kyoto University, Kyoto, Japan.
- Leister, D, 2004. Tandem and segmental gene duplication and recombination in the evolution of plant disease resistance genes. Trends Genet. 20 116–122. [DOI] [PubMed] [Google Scholar]
- Lubbers, E. L., K. S. Gill, T. S. Cox and B. S. Gill, 1991. Variation of molecular markers among geographically diverse accessions of Triticum tauschii. Genome 34 354–361. [Google Scholar]
- Mauricio, R., E. A. Stahl, T. Korves, D. Tian, M. Kreitman et al., 2003. Natural selection for polymorphism in the disease resistance gene Rps2 of Arabidopsis thaliana. Genetics 163 735–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden, E. S., and E. R. Sears, 1946. The origin of Triticum spelta and its free-threshing hexaploid relatives. J. Hered. 37 81–89, 107–116. [DOI] [PubMed] [Google Scholar]
- McIntosh, R. A., C. R. Wellings and R. F. Park, 1995. Wheat Rusts: An Atlas of Resistance Genes. Kluwer Academic Publishers, Boston.
- Michelmore, R. W., and B. C. Meyers, 1998. Clusters of resistance genes in plants evolve by divergent selection and a birth-and-death process. Genome Res. 8 1113–1130. [DOI] [PubMed] [Google Scholar]
- Nesbitt, M., and D. Samuel, 1998. Wheat domestication: archaeobotanical evidence. Science 279 1431.9508710 [Google Scholar]
- Qi, L. L., B. Echalier, S. Chao, G. R. Lazo, G. E. Butler et al., 2004. A chromosome bin map of 16,000 expressed sequence tag loci and distribution of genes among the three genomes of polyploid wheat. Genetics 168 701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raupp, W. J., L. E. Browder and B. S. Gill, 1983. Leaf rust resistance in Aegilops squarrosa: its transfer and expression in common wheat (Triticum aestivum L.). Phytopathology 73 818. [Google Scholar]
- Rose, L. E., P. D. Bittner Eddy, C. H. Langley, E. B. Holub, R. W. Michelmore et al., 2004. The maintenance of extreme amino acid diversity at the disease resistance gene, RPP13, in Arabidopsis thaliana. Genetics 166 1517–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland, G. G., and E. R. Kerber, 1974. Telocentric mapping in hexaploid wheat of genes for leaf rust resistance and other characters derived from Aegilops squarrosa. Can. J. Genet. Cytol. 16 137–144. [Google Scholar]
- Spielmeyer, W., O. Moullet, A. Laroche and E. S. Lagudah, 2000. Highly recombinogenic regions at seed storage protein loci on chromosome 1DS of Aegilops tauschii, the D-genome donor of wheat. Genetics 155 361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl, E. A., G. Dwyer, R. Mauricio, M. Kreitman and J. Bergelson, 1999. Dynamics of disease resistance polymorphism at the Rpm1 locus of Arabidopsis. Nature 400 667–671. [DOI] [PubMed] [Google Scholar]
- Talbert, L. E., L. Y. Smith and N. K. Blake, 1998. More than one origin of hexaploid wheat is indicated by sequence comparison of low-copy DNA. Genome 41 402–407. [Google Scholar]