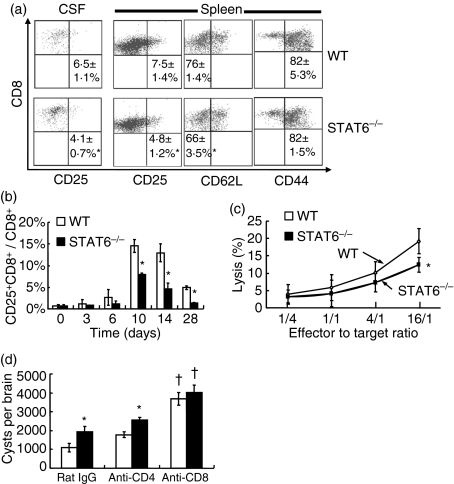
Figure 3.
Activation of CD8+ T cells after Toxoplasma gondii infection. (a) Cerebrospinal fluid (CSF) cells and splenocytes were taken from mice on day 28 post-infection (pi), and stained with fluorescein isothiocyanate (FITC)-anti-CD25, FITC-anti-CD62L, FITC-anti-CD44 and phycoerythrin (PE)-anti-CD8 monoclonal antibodies (mAbs) and then analysed by flow cytometry. Numbers indicate the percentage of CD25+ CD8+, CD62Llow CD8+ and CD44+ CD8+ T cells in CD8+ T cells in the CSF and spleen. Data are mean ± standard deviation (SD) (n = 4). (b) The percentage of CD25+ CD8+ T cells in CD8+ T cells in peripheral blood is shown. Data are mean ± SD (n = 5). (c) Purified CD8+ T cells and peritoneal macrophages labelled with PKH67 were co-cultured at various effector to target cell (E:T) ratios. Dead cells were determined by flow cytometry after staining with 7-amino-actinomycin D (7-AAD). Data are mean ± SD (n = 4). (d) Toxoplasma gondii-infected mice were intraperitoneally injected with 0·5 mg of anti-CD4 mAb, anti-CD8 mAb or rat immunoglobulin G (IgG) on days 14 and 21 pi. Cysts were counted on day 28 pi. Data are mean ± SD. (n = 5). Experiments were carried out three times with similar results. *Significantly different from WT mice (P <0·05). †Significantly different from rat IgG-treated mice (P <0·05).