Fig. 5.
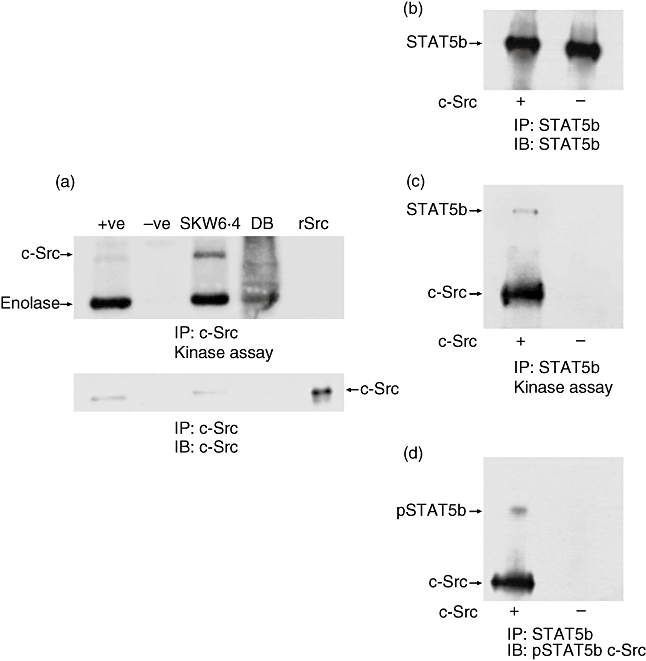
Signal transducer and activator of transcription 5b (STAT5b) is phosphorylated on tyrosine 699 by c-Src. DB cells that do not contain c-Src kinase were used to immunoprecipitate STAT5b followed by addition of human recombinant c-Src (rSrc) in a kinase assay. (a) 50 × 106 DB cells and 5 × 106 SKW 6·4 control cells were immunoprecipitated with anti-c-Src (monoclonal antibody 327) bound to protein A-Sepharose beads and a kinase assay performed with rabbit muscle enolase as exogenous substrate (a: top part); +ve: rSrc and −ve: just anti-c-Src bound to protein A-Sepharose without lysate. Thre lower part of the figure shows a Western blot using anti-c-Src (GD11). (b) 5 × 106 DB cells were used to immunoprecipiate STAT5b followed by a kinase assay using rSrc without rabbit muscle enolase. A Western blot was performed showing that STAT5b was immunoprecipitated. (c) The kinase results of the same experiment show phosphorylation of only the STAT5b and the autophosphorylation of the rSrc. (d) The membrane was stripped and reprobed using anti-pSTAT5b and anti-c-Src, to control for the Western blot.
