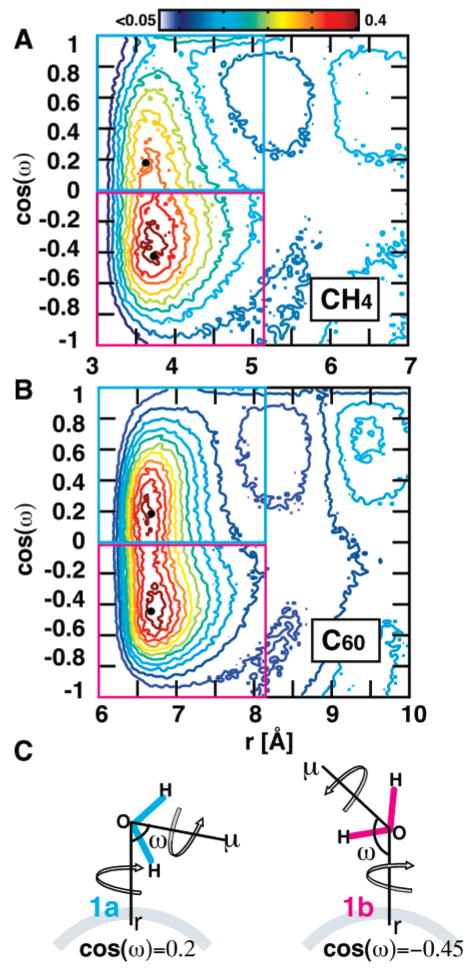
Figure 3.
Two-dimensional radial distribution function, gsol–O(r,cos(ω)), in part A for CH4 and in part B for C60. Water molecules in shell 1 (S1) tend to arrange tangentially around both solutes at a distance from the surface of about 1 Å and centered at a tilt angle of ω = 100° (cos(ω) = −0.43) and peak height of 0.39 for CH4 and centered at a tilt angle of ω = 117° (cos(ω) = −0.45) and peak height 0.28 for C60. Both solutes have a second population of water molecules in S1 with an inward orientation. This orientation is more populated in C60, centered at ω = 79° (cos(ω) = 0.19) and a peak height of 0.37. We observe that waters near a small highly curved surface adopt the narrower distribution with a preference for the tangential orientation, while waters near a larger surface adopt a broad distribution. (C) A schematic showing how (r,ω,μ) are defined. We further classify the water molecules in S1 according to their orientation to the solute. Water molecules that point the water dipole toward the solute are denoted as “1a”, and water molecules that point the water dipole away are denoted as “1b”. It is important to note that the tilt angle is unaffected by rotation about the μ and r vectors.