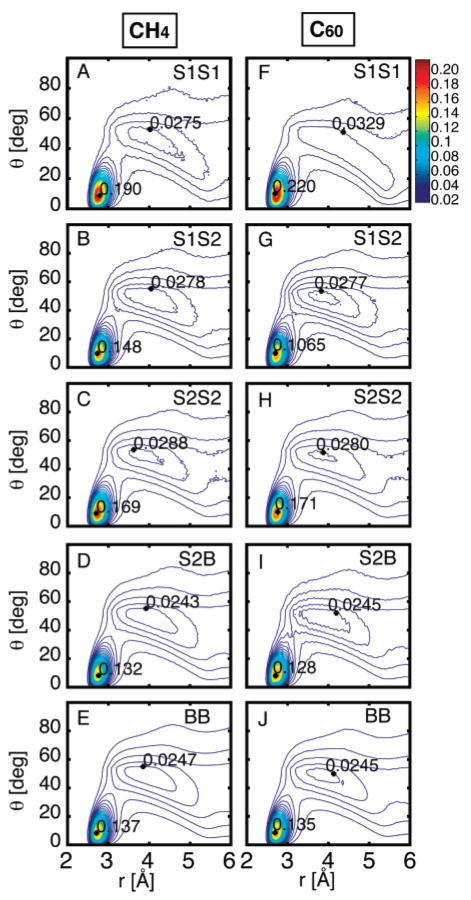
Figure 4.
Water···water pairwise interactions are characterized by the O···O distance in Å and the smallest O···OH angle in degrees. The radial distribution function g(r,θ) shows the water···water contact length/angle distribution, and these pairwise interactions are classified by shell. The distributions g(r,θ) of interactions between S1···S1, S1···S2, S2···S2, S2···B, and B···B are shown in parts A–E for C60 and in parts F–J for CH4. The first sharp peak at (r = 2.7 Å, θ = 10°) corresponds to a “good” linear hydrogen-bonding geometry, and the second broad peak at (r = 4.8 Å, θ = 45°) corresponds to close O···O pairs that are in contact but are not hydrogen bonded. There is an increase in hydrogen-bonded waters within S1, particularly for C60, and a disruption of hydrogen bonding between S1···S2 contacts.