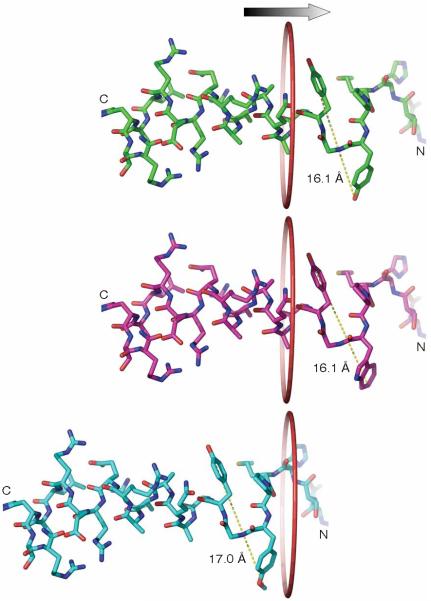
Figure 6.
Diameter of the inactivation peptide affects channel inactivation owing to the size restriction of the side portal. Upper panel, NMR structure of the inactivation peptide from Asn14 to Arg37 of Kv1.4. The central distance between the oxygen atoms of the hydroxyl groups of Tyr19 and Tyr21 is 16.1 Å. The surface distance of these two residues is thus ~19.9 Å by including the Van der Waals radius of the hydrogen atom at both ends. Trp (middle panel) and OmeTyr (bottom panel) were modeled in the structure and the corresponding central distances were measured. A ring with a diameter of 20 Å is drawn to illustrate the side portal entrance. The change of Tyr19 to OmeTyr increases the distal distance by ~0.9 Å, which can impede the entry of residue 19 and following residues of the inactivation peptide into the portal, effectively abolishing the fast inactivation.