Abstract
While dendritic cell (DC) vaccines can protect hosts from tumor challenge, their ability to effectively inhibit the growth of established tumors remains indeterminate. Previously, we have shown that human DCs transduced with Venezuelan equine encephalitis virus replicon particles (VRPs) were potent stimulators of antigen-specific T cells in vitro. Therefore, we investigated the ability of VRP-transduced DCs (VRP-DCs) to induce therapeutic immunity in vivo against tumors overexpressing the neu oncoprotein. Transduction of murine DCs with VRPs resulted in high-level transgene expression, DC maturation and secretion of proinflammatory cytokines. Vaccination with VRP-transduced DCs (VRP-DCs) expressing a truncated neu oncoprotein induced robust neu-specific CD8+ T cell and anti-neu IgG responses. Furthermore, a single vaccination with VRP-DCs induced the regression of large established tumors in wild-type mice. Interestingly, depletion of CD4+, but not CD8+, T cells completely abrogated inhibition of tumor growth following vaccination. Taken together, our results demonstrate that VRP-DC vaccines induce potent immunity against established tumors, and emphasize the importance of the generation of both CD4+ T cell and B cell responses for efficient tumor inhibition. These findings provide the rationale for future evaluation VRP-DC vaccines in the clinical setting.
Keywords: Dendritic cells, tumor immunotherapy, viral vectors, c-erbB-2, alphaviruses
1. Introduction
Vaccination is an attractive approach for treating metastatic cancer, as this strategy would not only eliminate malignant cells but also prevent recurrent disease through establishment of immunological memory [1]. Because of their role in initiating adaptive immune responses, dendritic cells (DCs) expressing tumor-associated antigens are increasingly utilized as therapeutic cancer vaccines [2]. While DC vaccines have proven highly efficacious in preventing tumor growth in several preclinical animal models, they have generally failed to consistently demonstrate objective therapeutic responses in cancer patients [3–8]. One explanation for this discrepancy may be that animal studies have primarily focused on prophylactic vaccination, whereas clinical trials typically involve therapeutic vaccination against established metastatic disease. The microenvironment of established tumors presents a formidable barrier for antitumor immune responses. Malignant cells can express cell surface molecules or secrete soluble mediators that directly inhibit NK or T cells [9]. In addition, tumor stromal cells can produce immunosuppressive cytokines and secrete chemokines that recruit regulatory cells to the tumor site, resulting in inhibition of tumor immunity [10]. Therefore, successful vaccination strategies must induce a robust immune response capable of overcoming immunoregulatory mechanisms present within established tumors.
The limited efficacy of DC vaccines at inducing therapeutic immune responses suggests that current methods for activation and antigen loading of DCs are suboptimal. Our group has argued that transduction of DCs with recombinant viral vectors may be an effective strategy for augmenting vaccine efficacy. Viral vectors can efficiently deliver tumor antigens to DCs in the context of an immunostimulatory viral infection, resulting in optimal DC activation [11–14]. Moreover, viral vectors may provide the persistent TLR stimulation deemed necessary for overcoming Treg-mediated suppression and thus breaking tolerance against tumor antigens [15]. We previously found that Venezuelan equine encephalitis virus replicon particles (VRP) could efficiently transduce human DCs, resulting in maturation and secretion of proinflammatory cytokines [14]. Furthermore, VRP-transduced DCs (VRP-DCs) were superior to peptide-pulsed DCs at stimulating the expansion of antigen-specific CTL populations in vitro, arguing that VRP-DCs may be an ideal vaccine for inducing robust tumor immunity. Based upon these prior results, we sought to examine the efficacy of VRP-DCs as therapeutic vaccines against the neu oncoprotein, an antigen frequently overexpressed by breast and ovarian carcinomas.
2. Materials and Methods
2.1 Mice, cell lines and peptides
FVB/N mice (H2q haplotype) were purchased from Jackson Laboratories (Bar Harbor, ME). Female mice (age 6–12 weeks) were used for all experiments. All animal experiments were performed in accordance with protocols approved by the University of North Carolina Institutional Animal Care and Use Committee. NIH-3T3 (American Type Culture Collection), 3T3neu and NT2 cells have been described [16]. RNEU420–429 (PDSLRDLSVF) and NP118–126 (RPQASGVYM) peptides were purchased from New England Peptide (Gardner, MA). RNEU420–429 is the immunodominant H2-Dq-restricted epitope from rat neu [17], while NP118–126 peptide is an H2-Dq-restricted epitope from the lymphocytic choriomeningitis virus nucleoprotein.
2.2 Generation of VRP-DC vaccines
VRPs encoding GFP (GFP-VRP) or VRPs lacking a functional transgene (null-VRP) have been described [18]. VRPs encoding the extracellular-transmembrane domains (amino acids 1–697) of rat neu (neuET-VRP) were generated by cloning a neuET cDNA into the pVR21 replicon plasmid [14]. VRP titer was determined by infection of baby hamster kidney (BHK) cells [14]. All VRPs were packaged in the wild type (V3000) viral envelope.
DCs were derived from bone marrow progenitor cells in the presence of GM-CSF and IL-4 [19]. On day 7 of culture, immature DCs were harvested and cryopreserved in 90% FBS/10% DMSO. DCs were stored in liquid nitrogen and used within three months of cryopreservation. To generate VRP-transduced DC (VRP-DC) vaccines, cryopreserved DCs were thawed at 37°C and washed twice with RPMI-10 media (RPMI-1640, 10% FBS, 2 mM L-glutamine, 50 μM 2-ME, 100 U/ml penicillin, 100 μg/ml streptomycin sulfate). DCs were plated in 6-well ultra low attachment plates at 106 cells/ml in RPMI-10 media supplemented with 5 ng/ml GM-CSF and IL-4, and cultured overnight at 37°C/5% CO2. The next morning, DCs were washed, suspended in RPMI-1H infection media (RPMI-1640, 1% FBS, 10mM HEPES) and plated at 106 cells/well in 6-well ultra low attachment plates. DCs were infected with VRP at a multiplicity of infection (MOI) of 10 for 2 hours at 37°C [14]. Infected DCs were washed three times and suspended in 0.9% sterile saline. Prior to vaccination, female FVB/N mice were anesthetized by intraperitoneal (i.p.) injection of 1.3 mg ketamine HCl/0.38 mg xylazine. VRP-DCs (106) were injected subcutaneously (s.c.) in the right axillary mammary gland adjacent to established tumors.
2.3 Antibodies and flow cytometric analysis
Monoclonal antibodies were purchased from eBioscience (San Diego, CA). The methods for flow analysis have been described previously [14]. Anti-c-ErbB2/neu (Ab4) monoclonal antibody was purchased from Calbiochem (San Diego, CA). PE-conjugated H-2Dq/RNEU420–429 tetramers were synthesized by the NIH Tetramer Facility (Emory University, Atlanta, GA). For tetramer staining, lymphocytes were incubated with PE-conjugated H-2Dq/RNEU420–429 tetramers (1:200) for 1 hour at room temperature; anti-CD8, anti-CD3 and anti-CD62L antibodies were added during the last 15 min of incubation. Cells were washed and suspended in 0.5% formaldehyde prior to analysis. Quantification of CD8+ T cells specific for RNEU420–429 was performed by intracellular IFN-γ staining as previously described [20].
2.4 Cytokine secretion assays
Murine DCs were infected with GFP-VRP (MOI =10), washed and plated into 96-well tissue-culture plates at 105 cells/well in a total volume of 200 μl of media with 5 ng/ml GM-CSF and IL-4. Analysis of IL-6, TNF-α and IL-10 was performed using the Murine Inflammation Cytometric Bead Array kit (BD Pharmingen). Analysis of IL-12p70 was performed using the BD OptEIA™ Mouse IL-12p70 ELISA Set (BD Pharmingen). Analysis of IFNα/β was determined by a type I interferon (IFN) bioassay [21].
2.5 Detection of serum anti-neu IgG
3T3 or 3T3neu cells were blocked with 20 μg/ml goat IgG (Sigma) for 15 min at 4°C. The cells were stained with two-fold dilutions of serum from vaccinated FVB/N mice for 1 hour at 4°C. Cells were washed twice and stained with goat anti-mouse IgG-FITC (Sigma) at a 1:200 dilution for 30 min at 4°C. Cells were washed twice and suspended in 1% formaldehyde. The median FITC fluorescence intensity (MFI) was measured using a Guava EasyCyte cell analysis system (Guava Technologies, Hayward, CA). Specific staining of neu was determined by subtracting the MFI of 3T3 cells from the MFI of 3T3neu cells. The concentration of neu-specific IgG in sera was calculated using a standard curve generated with Ab4 monoclonal antibody.
2.6 Therapeutic vaccination with VRP-DC
For each tumor challenge experiment, a fresh vial from the same lot of cryopreserved NT2 tumor cells was thawed and passaged in vitro for 5–10 days. NT2 cells were harvested, washed, and suspended in HBSS. FVB/N mice were challenged with 2 × 106 syngeneic NT2 cells s.c. in the mammary fat pad. Tumors were allowed to establish and grow for 7 days, at which time mice were vaccinated with VRP-DCs. Tumor area was measured twice weekly with metric calipers. Mice were sacrificed when tumor area was >200mm2.
2.7 In vivo depletion of lymphocytes
CD4+ or CD8+ T cells were depleted by i.p. injection of 0.5 mg of GK1.5 or 53.6.72 mAb (Bio Express, West Lebanon, NH), respectively as previously described [16]. Control mice received i.p. injections of 0.5 mg rat IgG (Sigma). Depletion of specific T cell populations was verified by flow analysis of splenocytes from treated mice (data not shown).
2.8 Isolation of tumor-infiltrating lymphocytes (TILs)
Tumors from vaccinated mice were excised, disrupted with a razor blade, and incubated under constant agitation for 1 hour with collagenase A (2.5 mg/ml), DNase I (17 μg/ml) and glass beads at 37°C. Undigested material was removed using a 100 μm nylon cell strainer. The single-cell suspension was suspended in 44% Percoll (Sigma), layered on a Lympholyte-M density gradient (Cedarlane Laboratories, Hornby, ON, Canada), and centrifuged for 30 minutes at 2500 rpm, 25°C. TILs at the Percoll-Lympholyte interface were removed and washed. For intracellular cytokine staining, TILs were stimulated for 4 hours with PMA (5 ng/ml) and ionomycin (500 ng/ml), and stained for surface expression of CD4 and CD8. Cells were fixed, permeabilized, and stained for intracellular expression of IFN-γ.
2.9 Statistical analysis
Statistical differences for costimulatory molecule expression, cytokine expression, and T cell and antibody responses were calculated by a two-tailed Student’s t test. Differences in survival were determined by Kaplan-Meier survival analysis. All statistical analyses were performed with GraphPad Prism® 3.0 software. For all analyses, a P value ≤ 0.05 was considered significant.
3. Results
3.1 Characterization of VRP-DC vaccines
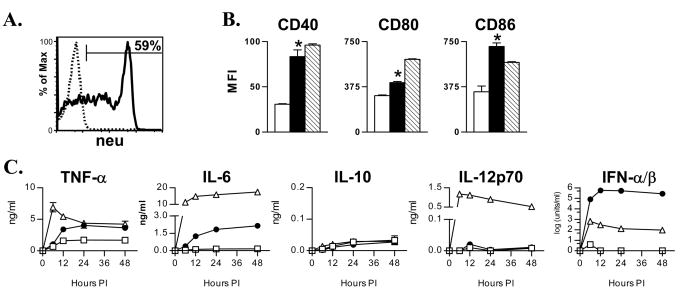
We initially investigated the ability of VRPs to transduce murine bone marrow-derived DCs in vitro. Peak expression of GFP in murine DCs occurred 6–12 hours following transduction with GFP-VRP (data not shown), which was similar to that found using human monocyte-derived DCs [14]. We next determined if murine DCs could be efficiently transduced with a VRP encoding neuET. VRP transgene expression peaked at 12 hours post-infection, with 59% of murine DC expressing high levels of neuET as determined by staining for surface and intracellular expression of the antigen (Figure 1A). Similar to our previous findings with human DCs, transgene expression decreased significantly by 36–48 hours post-infection [14]. The decrease in VRP transgene expression was accompanied by an increase in annexin-V expression as determined by flow cytometry, indicating that VRP-transduced DCs undergo accelerated apoptosis (data not shown).
Figure 1. VRP transduction results in high-level transgene expression and DC activation.
A. DCs were transduced with neuET-VRP (MOI = 10) and analyzed 12 hours later for expression of neuET by FACS. B. DCs were mock-infected (open bars), infected with GFP-VRP (solid bars), or treated with 100 ng/ml LPS (hatched bars). 24 hours later, DCs were stained for CD40, CD80, and CD86. Expression of costimulatory molecules on VRP-infected DCs was determined by gating on GFP+ DCs. Bars represent the mean +/− SEM (n = 4 per group). One of four similar experiments is depicted. C. DCs were mock-infected (open squares), infected with GFP-VRP (closed circles), or treated with 100 ng/ml LPS (open triangles). Cytokine levels were evaluated at the indicated times post-infection as described in Materials and Methods. *p<0.001 v. mock-DCs, Student’s t test.
We characterized the ability of VRPs to induce DC activation. VRP-DCs exhibited increased expression of the costimulatory molecules CD40, CD80 and CD86 in comparison to mock-infected DCs (Figure 1B). VRP-induced expression of CD40 and CD86 was comparable to that observed following treatment with LPS, whereas induction of CD80 by VRPs was slightly less than that seen with LPS. We also evaluated cytokine secretion by DCs following VRP infection (Figure 1C). VRP-transduced DCs secreted greater quantities of TNF-α, IL-6 and IFN-α/β compared to mock-infected DCs. LPS treatment induced more IL-6 secretion than VRP infection, while neither induced appreciable IL-10 production. Interestingly, VRP infection of murine DCs did not induce substantial IL-12p70 secretion, which is in contrast to our previous studies with human DCs [14]. However, VRP infection was a potent inducer of type I IFN, as VRP-transduced DCs secreted nearly 1000-fold more IFN-α/β than LPS-treated DCs. DCs treated with VRP that had been inactivated with ultraviolet light were similar to mock-infected DCs in terms of phenotype and cytokine secretion (data not shown), indicating that the effects of VRP infection were not due to a contaminant in the VRP preparation. Overall, VRP infection of murine DCs resulted in high-level transgene expression, phenotypic maturation, and secretion of proinflammatory cytokines—important characteristics predictive of a potent DC vaccine.
3.2 VRP-DC vaccines induce potent T cell and antibody responses against neu
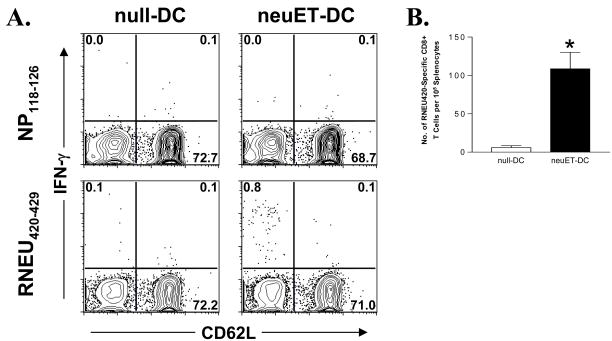
We next assessed the immunogenicity of VRP-DC vaccines in vivo. FVB/N mice were immunized with DCs transduced with neuET-VRP (neuET-DCs), and similarly boosted 14 days later. At seven days post-boost, spleens were harvested and evaluated for neu-specific CD8+ T cells by intracellular IFN-γ staining. Mice vaccinated with neuET-DCs, but not with DCs transduced by vector alone (null-DCs), had a significant population of CD8+CD62L− cells specific for the immunodominant peptide RNEU420–429 (Figure 2A–B).
Figure 2. Vaccination with VRP-DCs expressing neuET induces neu-specific CD8+ T cells.
FVB/N mice (n = 6 mice per group) were vaccinated with 106 null- or neuET-DCs, and similarly boosted two weeks later. At seven days post-boost, splenocytes were isolated and stimulated with either RNEU420–429 peptide or irrelevant NP118–126 peptide and assayed for intracellular IFN-γ expression. A. Representative staining of gated CD3+CD8+ T cells from vaccinated mice. B. The frequency of RNEU420–429-specific CD8+ T cells was determined by subtracting the frequency of NP118–126 specific T cells. Bars represent the mean +/− SEM. Data is representative of two experiments.
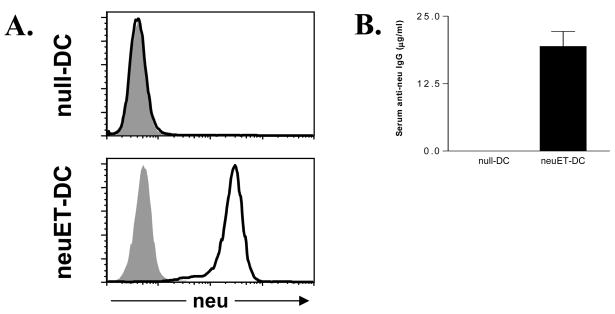
A shortcoming of traditional peptide-pulsed DC vaccines is their inability to induce antibody responses. Because humoral immunity is important for controlling the growth of neu-expressing tumors [22], we evaluated the ability of neuET-DC vaccines to induce neu-specific antibody responses. neu-specific IgG was readily detectable in the sera from mice vaccinated with neuET-DCs but not with null-DCs (Figure 3A). Quantification of neu-specific IgG demonstrated significant levels in mice vaccinated with neuET-DCs, whereas anti-neu IgG was undetectable in mice vaccinated with null-DCs (Figure 3B). Taken together, these results indicate that VRP-DC vaccines have the capacity to induce both humoral and cellular immunity against neu in vivo.
Figure 3. VRP-DC vaccines stimulate robust serum levels of neu-specific IgG.
FVB/N mice (n = 6 mice per group) were vaccinated with 106 null- or neuET-DCs, and similarly boosted two weeks later. At seven days post-boost, sera was harvested and used to stain 3T3 cells or 3T3neu cells, followed by staining with goat anti-mouse IgG-FITC. A. Representative staining of 3T3 cells (shaded histogram) or 3T3neu cells (heavy line) with sera from mice vaccinated with either null-DCs or neuET-DCs. B. Quantification of serum anti-neu IgG in vaccinated mice. Bars represent the mean +/− SEM. *p<0.001, Student’s t test.
3.3 VRP-DC vaccines induce therapeutic immunity against established tumors
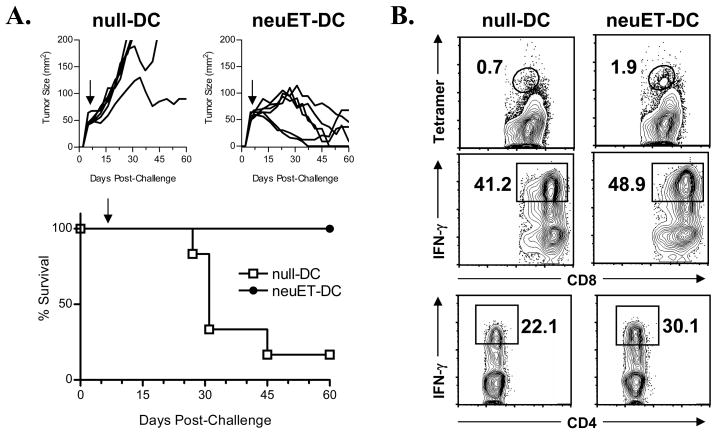
While DC vaccines can frequently protect mice from subsequent tumor challenge or spontaneous tumor development [12, 13], few studies have evaluated their efficacy at inducing therapeutic immunity in mice with preexisting tumors [11, 23, 24]. The ability of DC vaccines to inhibit the growth of established tumors is more relevant for determining clinical efficacy, as the majority of patients to be vaccinated have existing disease. Therefore, we evaluated the ability of VRP-DC vaccines to inhibit the growth of established tumors in FVB/N mice. Mice were challenged with NT2 tumor cells and vaccinated with neuET-DCs seven days later when tumor size was approximately 50 mm2. A single vaccination with neuET-DCs resulted in inhibition of tumor growth, and induced regression in the majority of treated mice (Figure 4, upper panels). Vaccination with neuET-DCs significantly prolonged survival (p=0.005) of tumor-bearing FVB/N mice when compared to vaccination with null-DCs (Figure 4, lower panel).
Figure 4. Therapeutic VRP-DC vaccination of tumor-bearing FVB/N mice inhibits tumor growth and induces tumor-infiltrating effector T cells.
A. FVB/N mice (n = 6 mice per group) were challenged with 2 × 106 NT2 tumor cells on day 0. Seven days later, mice received a single vaccination of 106 null- or neuET-DCs (solid arrow) and were evaluated for tumor growth (upper figures) and survival (lower figure). Mice vaccinated with neuET-DC demonstrated a significantly prolonged survival (p = 0.005, Kaplan-Meier survival analysis). One of two similar experiments is shown. B. TIL were isolated at 21 days post-vaccination. neu-specific CD8+ T cells were determined by staining with H2-Dq/RNEU420–429 tetramers. For assessment of IFN-γ production, TIL were stimulated for 4 h with PMA/ionomycin prior to intracellular IFN-γ staining.
Because effective tumor immunity has been associated with increased lymphocytic infiltration of tumors [25], we characterized the TILs isolated from the tumor site (Figure 4B). Mice vaccinated with neuET-DCs had an increased percentage of RNEU420–429-specific CD8+ T cells as determined by tetramer staining in comparison to mice receiving null-DCs. Mice vaccinated with neuET-DCs also had an increase in the percentages of IFN-γ+ CD8+ and CD4+ T cells at the tumor site, indicating a possible mechanism for tumor clearance.
3.4 CD4+ T cells are critical for inhibition of established tumors following VRP-DC vaccination
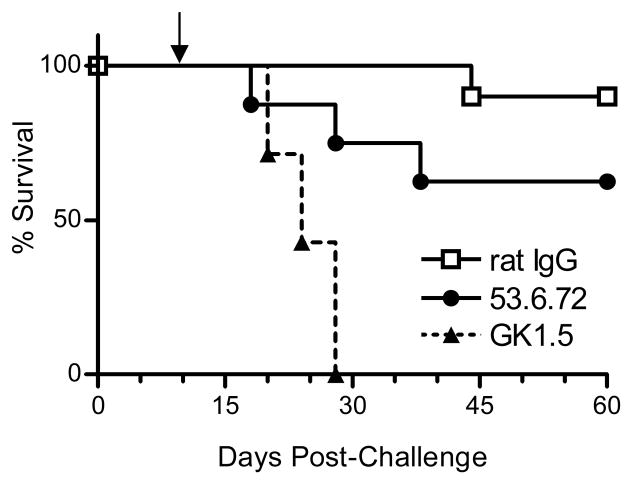
The increased numbers of neu-specific CD8+ TIL in mice vaccinated with neuET-DCs suggested that CD8+ T lymphocytes may be the primary effector cells responsible for controlling the growth of established tumors. To more specifically evaluate the role of different T cell populations in mediating inhibition of tumor growth, we depleted mice of either CD4+ or CD8+ T cells prior to vaccination (Figure 5). Treatment with GK1.5 or 53.6.72 monoclonal antibodies resulted in >99% depletion of splenic CD4+ or CD8+ T cells, respectively (data not shown). Interestingly, tumor immunity was only partially dependent upon CD8+ T cells, as 62.5% of CD8-depleted mice survived to day 60 post-tumor challenge. Conversely, we found that CD4+ T cells were crucial for effective tumor immunity, as all CD4-depleted mice experienced rapid tumor growth and had to be euthanized within four weeks of tumor-challenge (Figure 5). Taken together, the presence of tumor-specific CD8+ T cells does not appear to be an absolute requirement in our vaccination model, whereas CD4+ T cells are essential for inhibition of tumor growth.
Figure 5. CD4+ T cells are critical for inhibition of tumor growth following VRP-DC vaccination.
FVB/N mice (8–10 mice per group) were depleted of CD8 or CD4 T cells by treatment with 53.6.72 or GK1.5 mAb, respectively. Control mice received rat IgG. T cell-depleted mice were challenged with 2 × 106 NT2 cells and vaccinated with 106 neuET-DC seven days later (solid arrow). T cell depletions were maintained by injection of antibody every 4–5 days during monitoring of survival. Cumulative data from two experiments is shown. Survival of mice receiving rat IgG was significantly increased when compared to GK1.5-treated mice or 53.6.72-treated mice (p < 0.001 for both groups, Kaplan-Meier survival analysis).
4. Discussion
The optimal DC vaccine would not only present significant quantities of antigen, thus allowing optimal activation of lymphocytes, but would also produce immune-enhancing cytokines necessary for T cell differentiation into functional effector cells [2]. Our previous studies with human DCs demonstrated that VRP transduction of DCs resulted in cellular activation, secretion of proinflammatory cytokines, and efficient stimulation of antigen-specific T cells. Based on these findings, we sought to determine if VRP-DCs expressing a relevant tumor antigen could induce effective therapeutic immunity against established tumors.
VRP-transduced DCs have many putative characteristics of potent DC vaccines, namely high-level transgene expression, upregulation of costimulatory molecules and secretion of proinflammatory cytokines. VRPs could efficiently transduce >50% of DCs at an MOI of 10. In comparison, infection of DCs with adenoviral vectors at an MOI of 300 resulted in only 39.5% transduction efficiency [13]. While neu protein levels were high at 12 hours post-infection, transgene expression declined significantly between 36–48 hours post-infection, likely due to VRP-induced apoptosis. It is possible that vector-induced apoptosis could limit the therapeutic application of VRP-DCs, although our data suggests that the lifespan of VRP-DCs is sufficient for activation of B and T cells. It is conceivable that VRP-mediated apoptosis may actually facilitate T cell activation, as transduced DCs undergoing apoptosis can augment immune responses via cross-presentation of DC-associated antigens by endogenous APCs [26]. Regardless of the effects of VRP-induced apoptosis on T cell activation, it is clear that the limited lifespan of VRP- DCs did not preclude the development of anti-neu responses and therapeutic tumor immunity in our model (Figures 2–4).
VRP transduction of DCs resulted in phenotypic maturation and secretion of the proinflammatory cytokines TNF-α and IL-6, but not the immunosuppressive cytokine IL-10. VRP-infected DCs also secreted large amounts of type I interferons, which can augment tumor immunity by activating NK cell activity [27], enhancing cross-presentation of antigen to CD8+ T cells [28], and inducing clonal expansion of antigen-specific CD4+ and CD8+ T cells [29, 30]. While high-level secretion of type I interferons is typically associated with plasmacytoid DCs, myeloid DCs can become robust producers of IFN-α/β following direct infection with RNA viruses [31]. In contrast to our studies with human DCs, VRP transduction of murine DCs did not induce significant secretion of IL-12p70. IL-12p70 promotes the production of IFN-γ and development of TH1 immune responses [32], which are often deemed necessary for effective tumor immunity. Nevertheless, previous studies have shown that IL-12 production by DC vaccines is not necessary for induction of antigen-specific CTL responses in vivo [33], which is consistent with our observations that VRP-DC vaccines could induce neu-specific CD8+ T cells. It is possible that cytokines such as IFN-α may substitute for IL-12 in providing the signals necessary for T cell proliferation and survival following antigen-specific activation [34]. Although IL-12 production by VRP-DC vaccines does not appear to be required for activation of CD8+ T cells, IL-12p70 secretion could conceivably be enhanced either by codelivery of IL-12-expressing VRPs or by treatment with CD40L [12, 35].
VRP-DC vaccines not only induced neu-specific CD8+ T cells, but also stimulated antibody responses against neu in wild-type mice—an important advantage for this vaccine strategy. While most studies judge the effectiveness of DC vaccines solely on their ability to stimulate CD8+ T cells, both humoral and cell-mediated immunity are necessary for complete clearance of neu-overexpressing tumor cells [22].
Because the majority of breast cancer patients present with long-standing disease with multiple metastases, successful vaccine platforms must be capable of inducing robust immune responses against sizeable solid tumors. Established tumors are often resistant to both immunological and pharmacological therapies. Not only do stromal cells act as a direct barrier to cellular and humoral components of the immune system, but they can also secrete anti-inflammatory mediators that dampen immune responses within the tumor environment [10]. In the current study, we observed that therapeutic vaccination with VRP-DCs significantly inhibited the growth of established tumors, and even induced regression in the majority of animals. Tumor regression was associated with a significant infiltrate of both IFN-γ-producing CD4+ and CD8+ T cells at the tumor site. IFN-γ is known to have potent anti-tumor activity through direct action upon malignant cells and indirectly through effects on tumor stromal cells [36]; therefore the induction of IFN-γ-producing TIL may be essential for the efficacy of VRP-DC vaccines. The role of IFN-γ and other cytokines in mediating immunity against established tumors in our vaccination model is currently being evaluated.
Interestingly, we found that CD4+ T cells were more important than CD8+ T cells for tumor inhibition following VRP-DC vaccination. CD4+ T cells are likely necessary for augmenting the expansion and activity of tumor-specific CD8+ T cells and the development of anti-neu antibody responses. A similar dependency of neu-specific immunity upon CD4+ T cells has been found in other studies. The efficacy of a vaccine composed of DCs transduced with an adenoviral vector encoding neuET required the presence of CD4+ but not CD8+ T cells in neu-T transgenic BALB/c mice [13]. CD4+ T cells were necessary for clearance of tumors in FVB/N mice vaccinated with irradiated 3T3neu cells expressing GM-CSF, although CD8+ T cells also had an important role in this model [22]. Taken together, these studies suggest that cancer vaccines that activate both CD4+ and CD8+ T cells are likely to be more successful than those aimed solely at stimulating CTL responses.
While tumor and stromal cells can directly inhibit antitumor responses, suppression of tumor immunity by hematopeoitically-derived regulatory cells is also important for tumor immune evasion. Notably, CD4+CD25+ regulatory T cells and myeloid suppressor cells can be recruited to the tumor site, where they mediate tolerance against tumor antigens and dampen tumoricidal responses [37]. Studies have demonstrated that tolerance against tumor antigens can be overcome through activation of TLR signaling by viral vectors, which may explain the efficacy of VRP-DCs [15]. However, while VRP-DCs can inhibit the growth of established tumors in FVB/N mice, preliminary studies have failed to demonstrate tumor regression in transgenic mice tolerant to the neu antigen (T. Moran, R. Johnston, and J. Serody, unpublished observations). These preliminary findings suggest that viral activation of DCs alone may not be sufficient for effective tumor immunity under conditions of tolerance. The mechanisms responsible for inhibiting tumor immunity in tolerant animals are currently being explored in our laboratory.
In summary, VRPs encoding a tumor antigen could efficiently transduce murine DCs, resulting in maturation and proinflammatory cytokine production. VRP-DC vaccines were highly immunogenic in vivo, and were capable of inducing both cellular and humoral immunity against neu. Most importantly, a single vaccination with VRP-DCs expressing neuET induced regression of bulky tumors, demonstrating that VRP-transduced DCs are potent vaccines with the capacity to induce effective therapeutic immunity against established tumors in wild-type mice. Therefore, VRP-transduced DCs may prove to be an optimal vaccine for immunotherapy of metastatic disease in the clinical setting.
Acknowledgments
For technical assistance, we thank Martha Collier, Hans Seidel, Joseph Thompson, and Alan Whitmore. We thank the laboratory of Lishan Su for assistance using the Guava EasyCyte, the laboratory of Mark Heise for antibody reagents, Mark Wallet and Carmen Wong for procedural advice, the UNC Flow Cytometry Facility for FACS services and the NIH Tetramer Facility for provision of reagents. We thank Marion Couch for generously providing the 3T3neu cells. This work was supported by Grants P50–CA58223 (to J.S.S.) and R01–AI51990 (to R.E.J.) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Emens LA, Reilly RT, Jaffee EM. Breast cancer vaccines: maximizing cancer treatment by tapping into host immunity. Endocr Relat Cancer. 2005 Mar;12(1):1–17. doi: 10.1677/erc.1.00671. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005 Apr;5(4):296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 3.Zaks TZ, Rosenberg SA. Immunization with a peptide epitope (p369–377) from HER-2/neu leads to peptide-specific cytotoxic T lymphocytes that fail to recognize HER-2/neu+ tumors. Cancer Res. 1998 Nov 1;58(21):4902–8. [PubMed] [Google Scholar]
- 4.Brossart P, Wirths S, Stuhler G, Reichardt VL, Kanz L, Brugger W. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood. 2000 Nov 1;96(9):3102–8. [PubMed] [Google Scholar]
- 5.Knutson KL, Schiffman K, Disis ML. Immunization with a HER-2/neu helper peptide vaccine generates HER-2/neu CD8 T-cell immunity in cancer patients. J Clin Invest. 2001 Feb;107(4):477–84. doi: 10.1172/JCI11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kono K, Takahashi A, Sugai H, Fujii H, Choudhury AR, Kiessling R, et al. Dendritic cells pulsed with HER-2/neu-derived peptides can induce specific T-cell responses in patients with gastric cancer. Clin Cancer Res. 2002 Nov;8(11):3394–400. [PubMed] [Google Scholar]
- 7.Murray JL, Gillogly ME, Przepiorka D, Brewer H, Ibrahim NK, Booser DJ, et al. Toxicity, immunogenicity, and induction of E75-specific tumor-lytic CTLs by HER-2 peptide E75 (369–377) combined with granulocyte macrophage colony-stimulating factor in HLA-A2+ patients with metastatic breast and ovarian cancer. Clin Cancer Res. 2002 Nov;8(11):3407–18. [PubMed] [Google Scholar]
- 8.Dees EC, McKinnon KP, Kuhns JJ, Chwastiak KA, Sparks S, Myers M, et al. Dendritic cells can be rapidly expanded ex vivo and safely administered in patients with metastatic breast cancer. Cancer Immunol Immunother. 2004 Sep;53(9):777–85. doi: 10.1007/s00262-004-0520-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khong HT, Restifo NP. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nature immunology. 2002 Nov;3(11):999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu P, Rowley DA, Fu YX, Schreiber H. The role of stroma in immune recognition and destruction of well-established solid tumors. Curr Opin Immunol. 2006 Apr;18(2):226–31. doi: 10.1016/j.coi.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Specht JM, Wang G, Do MT, Lam JS, Royal RE, Reeves ME, et al. Dendritic cells retrovirally transduced with a model antigen gene are therapeutically effective against established pulmonary metastases. The Journal of experimental medicine. 1997 Oct 20;186(8):1213–21. doi: 10.1084/jem.186.8.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Emtage P, Zhu Q, Foley R, Muller W, Hitt M, et al. Induction of ErbB-2/neu-specific protective and therapeutic antitumor immunity using genetically modified dendritic cells: enhanced efficacy by cotransduction of gene encoding IL-12. Gene Ther. 2001 Feb;8(4):316–23. doi: 10.1038/sj.gt.3301396. [DOI] [PubMed] [Google Scholar]
- 13.Sakai Y, Morrison BJ, Burke JD, Park JM, Terabe M, Janik JE, et al. Vaccination by genetically modified dendritic cells expressing a truncated neu oncogene prevents development of breast cancer in transgenic mice. Cancer Res. 2004 Nov 1;64(21):8022–8. doi: 10.1158/0008-5472.CAN-03-3442. [DOI] [PubMed] [Google Scholar]
- 14.Moran TP, Collier M, McKinnon KP, Davis NL, Johnston RE, Serody JS. A novel viral system for generating antigen-specific T cells. J Immunol. 2005 Sep 1;175(5):3431–8. doi: 10.4049/jimmunol.175.5.3431. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Huang CT, Huang X, Pardoll DM. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nature immunology. 2004 May;5(5):508–15. doi: 10.1038/ni1059. [DOI] [PubMed] [Google Scholar]
- 16.Reilly RT, Gottlieb MB, Ercolini AM, Machiels JP, Kane CE, Okoye FI, et al. HER-2/neu is a tumor rejection target in tolerized HER-2/neu transgenic mice. Cancer Res. 2000 Aug 1;60(13):3569–76. [PubMed] [Google Scholar]
- 17.Ercolini AM, Machiels JP, Chen YC, Slansky JE, Giedlen M, Reilly RT, et al. Identification and characterization of the immunodominant rat HER-2/neu MHC class I epitope presented by spontaneous mammary tumors from HER-2/neu-transgenic mice. J Immunol. 2003 May 15;170(8):4273–80. doi: 10.4049/jimmunol.170.8.4273. [DOI] [PubMed] [Google Scholar]
- 18.Thompson JM, Whitmore AC, Konopka JL, Collier ML, Richmond EM, Davis NL, et al. Mucosal and systemic adjuvant activity of alphavirus replicon particles. Proc Natl Acad Sci U S A. 2006 Mar 7;103(10):3722–7. doi: 10.1073/pnas.0600287103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng-Cashin J, Kuhns JJ, Burkett SE, Powderly JD, Craven RR, van Deventer HW, et al. Host absence of CCR5 potentiates dendritic cell vaccination. J Immunol. 2003 Apr 15;170(8):4201–8. doi: 10.4049/jimmunol.170.8.4201. [DOI] [PubMed] [Google Scholar]
- 20.Ercolini AM, Ladle BH, Manning EA, Pfannenstiel LW, Armstrong TD, Machiels JP, et al. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. The Journal of experimental medicine. 2005 May 16;201(10):1591–602. doi: 10.1084/jem.20042167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White LJ, Wang JG, Davis NL, Johnston RE. Role of alpha/beta interferon in Venezuelan equine encephalitis virus pathogenesis: effect of an attenuating mutation in the 5′ untranslated region. Journal of virology. 2001 Apr;75(8):3706–18. doi: 10.1128/JVI.75.8.3706-3718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reilly RT, Machiels JP, Emens LA, Ercolini AM, Okoye FI, Lei RY, et al. The collaboration of both humoral and cellular HER-2/neu-targeted immune responses is required for the complete eradication of HER-2/neu-expressing tumors. Cancer Res. 2001 Feb 1;61(3):880–3. [PubMed] [Google Scholar]
- 23.Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Celluzzi C, Falo LD, et al. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med. 1995 Dec;1(12):1297–302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 24.Song W, Kong HL, Carpenter H, Torii H, Granstein R, Rafii S, et al. Dendritic cells genetically modified with an adenovirus vector encoding the cDNA for a model antigen induce protective and therapeutic antitumor immunity. The Journal of experimental medicine. 1997 Oct 20;186(8):1247–56. doi: 10.1084/jem.186.8.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002 Oct 25;298(5594):850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Racanelli V, Behrens SE, Aliberti J, Rehermann B. Dendritic cells transfected with cytopathic self-replicating RNA induce crosspriming of CD8+ T cells and antiviral immunity. Immunity. 2004 Jan;20(1):47–58. doi: 10.1016/s1074-7613(03)00353-4. [DOI] [PubMed] [Google Scholar]
- 27.Herberman RR, Ortaldo JR, Bonnard GD. Augmentation by interferon of human natural and antibody-dependent cell-mediated cytotoxicity. Nature. 1979 Jan 18;277(5693):221–3. doi: 10.1038/277221a0. [DOI] [PubMed] [Google Scholar]
- 28.Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D, et al. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nature immunology. 2003 Oct;4(10):1009–15. doi: 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- 29.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. The Journal of experimental medicine. 2005 Sep 5;202(5):637–50. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Havenar-Daughton C, Kolumam GA, Murali-Krishna K. Cutting Edge: The direct action of type I IFN on CD4 T cells is critical for sustaining clonal expansion in response to a viral but not a bacterial infection. J Immunol. 2006 Mar 15;176(6):3315–9. doi: 10.4049/jimmunol.176.6.3315. [DOI] [PubMed] [Google Scholar]
- 31.Diebold SS, Montoya M, Unger H, Alexopoulou L, Roy P, Haswell LE, et al. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature. 2003 Jul 17;424(6946):324–8. doi: 10.1038/nature01783. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi M, Fitz L, Ryan M, Hewick RM, Clark SC, Chan S, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. The Journal of experimental medicine. 1989 Sep 1;170(3):827–45. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan Y, Lu L, Bramson JL, Baral S, Zhu Q, Pilon A, et al. Dendritic cell-derived IL-12 is not required for the generation of cytotoxic, IFN-gamma-secreting, CD8(+) CTL in vivo. J Immunol. 2001 Nov 1;167(9):5027–33. doi: 10.4049/jimmunol.167.9.5027. [DOI] [PubMed] [Google Scholar]
- 34.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005 Apr 15;174(8):4465–9. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- 35.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. The Journal of experimental medicine. 1996 Aug 1;184(2):747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blankenstein T. The role of tumor stroma in the interaction between tumor and immune system. Curr Opin Immunol. 2005 Apr;17(2):180–6. doi: 10.1016/j.coi.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004 Sep;10(9):942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]