Abstract
Aging and obesity are characterized by decreased β-cell sensitivity and defects in the potentiation of nutrient-stimulated insulin secretion by GIP. Exercise and diet are known to improve glucose metabolism and the pancreatic insulin response to glucose, and this effect may be mediated through the incretin effect of GIP. The purpose of this study was to assess the effects of a 12-wk exercise training intervention (5 days/wk, 60 min/day, 75% V̇o2 max) combined with a eucaloric (EX, n = 10) or hypocaloric (EX-HYPO, pre: 1,945 ± 190, post: 1,269 ± 70, kcal/day; n = 9) diet on the GIP response to glucose in older (66.8 ± 1.5 yr), obese (34.4 ± 1.7 kg/m2) adults with impaired glucose tolerance. In addition to GIP, plasma PYY3–36, insulin, and glucose responses were measured during a 3-h, 75-g oral glucose tolerance test. Both interventions led to a significant improvement in V̇o2 max (P < 0.05). Weight loss (kg) was significant in both groups but was greater after EX-HYPO (−8.3 ± 1.1 vs. −2.8 ± 0.5, P = 0.002). The glucose-stimulated insulin response was reduced after EX-HYPO (P = 0.02), as was the glucose-stimulated GIP response (P < 0.05). Furthermore, after the intervention, changes in insulin (ΔI0–30/ΔG0–30) and GIP (Δ0–30) secretion were correlated (r = 0.69, P = 0.05). The PYY3–36 (Δ0–30) response to glucose was increased after both interventions (P < 0.05). We conclude that 1) a combination of caloric restriction and exercise reduces the GIP response to ingested glucose, 2) GIP may mediate the attenuated glucose-stimulated insulin response after exercise/diet interventions, and 3) the increased PYY3–36 response represents an improved capacity to regulate satiety and potentially body weight in older, obese, insulin-resistant adults.
Keywords: incretins, weight loss, insulin resistance, gut hormones
glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide (GLP-1) represent the primary incretin hormones and have been shown to stimulate insulin secretion in response to nutrient or glucose ingestion (4, 11, 14). Although GIP and GLP-1 share similar metabolic functions, they act through independent pathways, and their effects are mediated through unique G protein-coupled receptors (9, 33). GIP is secreted from K cells in the small intestine, and increased secretion has been reported in obesity (15, 31, 39), impaired glucose tolerance (IGT) (39), and type 2 diabetes mellitus (15). It has been suggested that this overactivation of the enteroinsular axis contributes to the hyperinsulinemia that develops secondary to insulin resistance in overweight/obese adults (15, 39). Transgenic mouse studies have revealed that targeted ablation of K cells reduces body weight and insulin resistance, thus suggesting that GIP may be an important therapeutic target for obesity and diabetes (2). Aging is also associated with GIP dysregulation (4, 28) as well as impaired β-cell sensitivity (7, 29, 38). Even healthy, older adults demonstrate a decreased β-cell sensitivity to GIP, which suggests that GIP may play a role in mediating the progressive impairment in glucose tolerance that characterizes aging (29). Further, genetic inactivation of GIP has been shown to prevent the development of aging-associated insulin resistance (44). Collectively, these observations suggest that GIP secretion and function represent an important regulatory mechanism for maintaining glucose homeostasis, and abnormalities in either secretion or function may be important determinants of obesity and diabetes.
Acute exercise and aerobic exercise training have been shown to affect incretin secretion (6, 20, 23, 34) and hormones related to energy balance (12, 18, 25). We and others have reported previously that acute exercise markedly attenuates the GIP response to glucose ingestion (6, 34). In highly trained athletes, this suppressed GIP response was accompanied by a corresponding reduction in insulin secretion (34). Furthermore, exercise training is known to reduce circulating insulin levels and improve the insulin response to hyperglycemia (20, 22). It has been suggested that the reductions in plasma insulin following exercise training may be due to modulation of the gastroenteropancreatic hormones (6, 23) that function primarily to maintain energy balance. Exercise creates a negative energy balance, and different mechanisms operate to maintain energy homeostasis: short-term regulation via gut hormones, including GIP and peptide tyrosine-tyrosine [peptide YY (PYY)3–36], and longer-term regulation via insulin. PYY is a satiety regulator, and plasma levels are abnormal in obesity (32, 45), thus suggesting that obesity adversely alters satiety peptides, thereby perpetuating a vicious cycle of weight gain and impaired peptide secretion and metabolic function. Although weight loss interventions (24, 32, 42) and bariatric surgery (24, 43) demonstrate that gut peptides respond to metabolic perturbations in obese persons, there are very limited data on the effects of exercise training (19, 23) on gut peptides and no data on exercise combined with calorie restriction in an insulin-resistant population such as the older obese.
Based on our previous observations that exercise can attenuate the GIP response to glucose in well-trained endurance athletes (34) and that exercise training enhances the insulin response to glucose in older adults (22), we sought to determine the effect of an exercise training intervention on GIP and its incretin effect on glucose-stimulated insulin secretion in older obese adults. Since weight loss may be an important factor in determining GIP release, we also assessed the effects of exercise training combined with a calorie-reduced diet on basal and glucose-stimulated gastroinsulinar peptides (GIP and PYY3–36). We hypothesized that exercise training plus a hypocaloric diet would reduce glucose-stimulated insulin and GIP release and increase PYY3–36 secretion, and because of the greater weight loss this improvement would be greater than exercise training alone.
METHODS
Participants.
Nineteen older obese men and women with IGT were recruited to participate in the study. Participants were classified as IGT if they had a fasting glucose that was ≥100 mg/dl but <126 mg/dl and a 2-h oral glucose tolerance test (OGTT) value between 140 and 199 mg/dl in accordance with the criteria of the American Diabetes Association (3). Medical screening included a complete history and physical examination, an electrocardiogram, an exercise stress test, and a complete metabolic blood profile. Individuals with diabetes, significant hypertension, and heart, liver, gastrointestinal, kidney, or pulmonary disease were excluded from the study. In addition, individuals with physical ailments that would have prevented them from exercising were also excluded. All participants were sedentary and weight stable for 6 mo prior to the study. All metabolic and physiological measurements were performed during a 3-day in-patient hospital stay in the General Clinical Research Center. The study was approved by the Institutional Review Board of the Cleveland Clinic, and all subjects provided written, informed consent in accordance with the guidelines for the protection of human subjects.
Intervention.
Subjects were randomized to one of two groups, an exercise training group that maintained its usual caloric intake [eucaloric diet (EX): n = 10, 8 males and 2 females; age: 65.7 ± 1.6 yr; BMI: 34.2 ± 1.8 kg/m2] or an exercise training group that was counseled to reduce its caloric intake by ∼500 kcal/day [hypocaloric diet (EX-HYPO): n = 9, 4 males and 5 females; age: 68.0 ± 1.4 yr; BMI: 34.5 ± 1.7 kg/m2]. Three-day diet records were collected prior to the beginning of the study, and individual nutritional counseling was provided weekly to reinforce and support the dietary restrictions and to monitor caloric intake. Exercise training included 50–60 min/day of supervised moderate-intensity aerobic exercise (treadmill/cycle ergometer) at ∼75% of maximal oxygen uptake capacity performed 5 days/wk (excluding weekends) for 12 wk.
Body composition.
Height without shoes was measured to the nearest 1.0 cm. Body weight was measured to the nearest 0.1 kg, with the subjects wearing only undergarments and a hospital gown. Waist circumference was measured at the level of the umbilicus. Body density was determined by hydrostatic weighing, and body fat mass was calculated using Siri equations, as described previously (35).
Aerobic fitness.
Physical fitness was measured using an incremental treadmill exercise test to determine subjects’ maximal oxygen consumption (V̇o2max), as described previously (40).
OGTT.
A 75-g OGTT was performed after a 12- to 14-h overnight fast pre- and postintervention. Postintervention, the OGTT was conducted within 18 h following the last exercise bout. Fasting baseline samples were drawn to determine initial glucose, insulin, and gut peptide measurements. Following baseline draws, a 75-g glucose drink was ingested within a 10-min period. Blood samples were drawn for glucose, insulin, and gut peptides at 30, 60, 90, 120, and 180 min after the glucose was ingested. Plasma glucose concentrations were measured immediately using the glucose oxidase method (Beckman Instruments, Fullerton, CA). For GIP and PYY3–36 analysis, blood was collected in vacutainers containing EDTA and the protease inhibitor aprotonin and was centrifuged at 1,000 rpm for 10 min at 4°C. The samples were stored at −80°C for subsequent analysis. An index of glucose-stimulated insulin secretion was calculated as insulin Δ0–30 min/glucose Δ0–30 min (1).
Blood analysis.
Insulin was assayed via commercial RIA (Linco, St. Charles, MO). Plasma GIP (total) and PYY3–36 were analyzed via commercial ELISA kits (Linco). To correct for interassay variability, all pre- and postmeasurements for each individual subject were run on the same plate.
Statistical analysis.
All values are expressed as means ± SE. Total hormonal responses (area under the curve) were calculated using the trapezoidal approach. In addition, immediate hormone secretory responses to glucose ingestion were estimated as Δ0–30 min. Repeated-measures ANOVA was used for between-group comparisons (Statview; SAS Institute, Cary, NC). Tukey post hoc tests were applied to significant group × time interactions. One-way ANOVA was used to compare baseline measurements; no differences were noted between the two groups for any variable. Correlation analyses were carried out to identify relationships created as a result of the intervention. Statistical significance was accepted at P < 0.05.
RESULTS
Dietary intake.
Based on weekly diet counseling with a research dietitian and 3-day diet records collected every 4 wk during the 12-wk intervention, we determined that those in the EX group consumed a diet similar (both caloric and macronutrient composition) to their prior usual diet (Table 1). The EX-HYPO group consumed ∼700 fewer kcal daily (Table 1), reduced fat intake by ∼5% [%kcal; preintervention (pre): 30 ± 1.3%, postintervention (post): 25 ± 1.1%], and increased carbohydrate intake by ∼6% (pre: 50.6 ± 2.6%, post: 56.9 ± 1.5%). The macronutrient composition was similar between the EX and EX-HYPO subjects both pre- and postintervention (Table 1).
Table 1.
Participant characteristics
| Variable | EX Group |
EX-HYPO Group | ||
|---|---|---|---|---|
| Preintervention | Postintervention | Preintervention | Postintervention | |
| Body weight, kg | 93.1±5.2 | 90.3±5.0* | 98.4±5.7 | 90.1±4.9*† |
| BMI, kg/m2 | 34.2±1.8 | 33.8±1.6* | 34.5±1.6 | 31.6±1.5*† |
| Fat mass, kg | 40.9±3.1 | 39.4±3.4* | 38.8±3.8 | 31.5±3.5*† |
| FFM, kg | 52.2±2.8 | 50.9±2.2 | 61.2±4.6 | 59.9±4.1 |
| V̇o2max, ml·kg FFM−1·min−1 | 34.1±1.1 | 38.2±1.6* | 36.1±1.7 | 39.9±1.5* |
| Caloric intake, kcal/day | 1,633±78 | 1,637±175 | 1,945±190 | 1,269±70* |
| Macronutrients, %carbohydrate/fat/protein | 48/34/18 | 46/34/20 | 51/30/19 | 57/25/18 |
Data represent means ± SE. Participant characteristics prior to and following 12 wk of aerobic exercise training and either a eucaloric (EX) or hypocaloric (EX-HYPO) diet. BMI, body mass index; FFM, fat-free mass. V̇o2max, maximal volume of oxygen consumption. EX, n = 10; EX-HYPO, n = 9.
Significantly lower than preintervention value, P < 0.05.
Significantly lower than the EX group, P < 0.05.
Body composition.
At the end of the 12-wk intervention, body weight (P < 0.0001), BMI (P = 0.0012), and fat mass (P < 0.0001) were significantly reduced in both groups (Table 1). As expected, subjects in the EX-HYPO group showed greater changes in these parameters (body weight, P = 0.002; BMI, P = 0.01; fat mass, P = 0.0008). Notably, there was no significant change in fat-free mass (FFM; P = 0.09) for either group.
Aerobic fitness.
V̇o2max (ml·kg FFM−1·min−1) was used as an index of aerobic fitness. Both groups achieved a significant improvement (P = 0.02) in V̇o2max over the course of the intervention (Table 1).
Glucose and insulin.
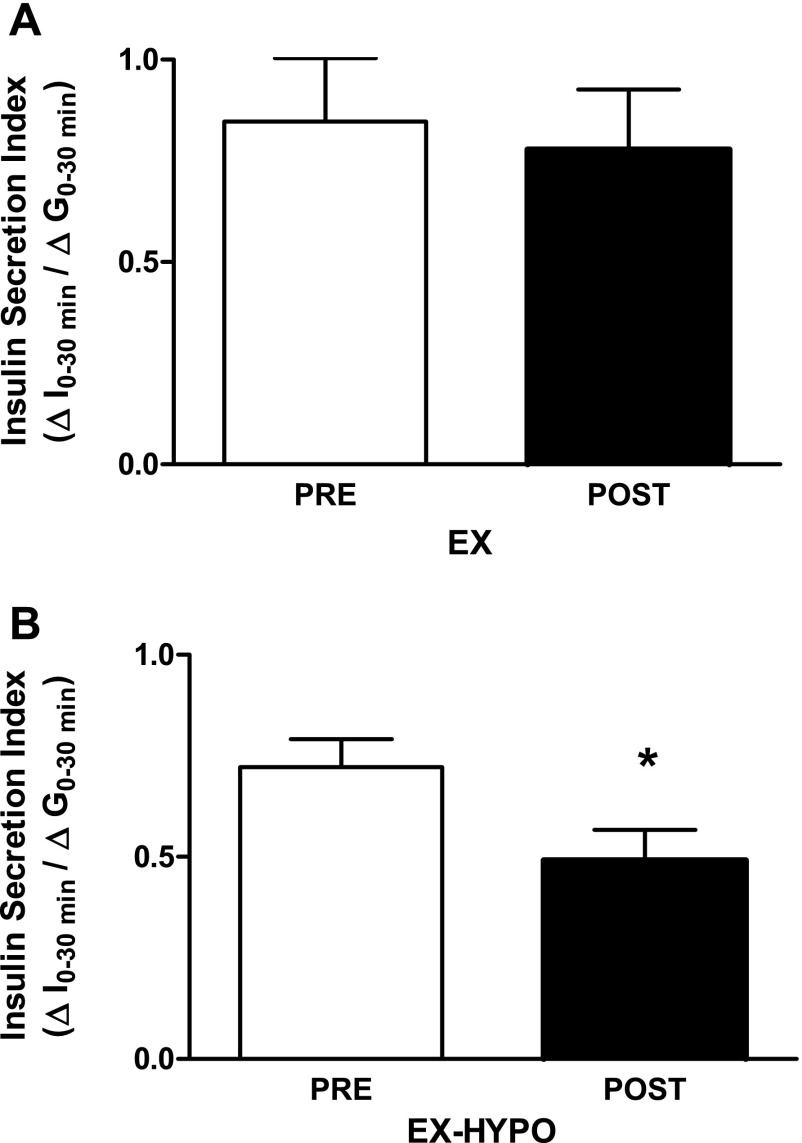
Fasting plasma glucose was significantly reduced after EX-HYPO (P = 0.04) but not after EX alone. Furthermore, the glucose response (area under the curve) was significantly reduced (P = 0.04) in the EX-HYPO group but not the EX group (Table 2). The EX-HYPO intervention induced a decrease in both fasting insulin (P = 0.04) and area under the insulin response curve (P = 0.01) (Table 2). EX alone was not sufficient to significantly reduce fasting insulin or the insulin response to glucose. Insulin secretion was significantly reduced in the EX-HYPO group following the intervention (pre: 0.86 ± 0.15, post: 0.55 ± 0.09; P = 0.03) and was decreased, but not significantly, in the EX group (pre: 0.85 ± 0.16; post: 0.78 ± 0.15) (Fig. 1, A and B). Insulin sensitivity was calculated using the Matsuda index, which is a function of fasting glucose and insulin, and the mean glucose and insulin responses during the OGTT (26). Insulin sensitivity was increased for EX-HYPO (pre: 2.69 ± 0.5, post: 4.16 ± 0.55; P = 0.01); however, there was no correlation between the change in insulin sensitivity and GIP (r = −0.335, P = 0.16).
Table 2.
Fasting glucose, insulin, and gut peptides and responses following glucose ingestion
| Variable | EX Group |
EX-HYPO Group | ||
|---|---|---|---|---|
| Preintervention | Postintervention | Preintervention | Postintervention | |
| Glucose, mg/dl | 112.3±5.8 | 110.2±4.5 | 112.0±4.7 | 100.5±4.2* |
| AUC, mg·dl−1·3 h | 30,903±1,670 | 29,183±1,220* | 28,629±1,478 | 25,281±1,477* |
| Insulin, μU/ml | 20.1±3.8 | 16.9±2.8 | 16.9±2.2 | 13.4±1.6*† |
| AUC, μU·ml−1·3 h | 11,724±2242 | 10,421±1,951 | 10,310±1,511 | 6,585±1,259* |
| GIP, pg/ml | 83.9±16 | 77.7±22 | 61.7±12 | 59.3±18 |
| AUC, pg·ml−1·3 h | 32,107±6976 | 37,314±6314 | 33,498±5,658 | 26,688±5,134† |
| PYY3−36, pg/ml | 43.3±6.9 | 39.7±2.7 | 43.1±5.0 | 40.1±6.9 |
| AUC, pg·ml−1·3 h | 7,843±895 | 7,542±1431 | 6,495±1400 | 6,994±2100 |
Data represent means ± SE. AUC, area under the curve; GIP, glucose-dependent insulinotropic polypeptide; PYY3−36, peptide YY3-36. Fasting plasma glucose, insulin, and gut peptide levels and plasma responses following oral glucose before and after the interventions. EX, n =10; EX-HYPO; n = 9.
Significantly lower than preintervention.
Significantly lower than the EX group, P < 0.05.
Fig. 1.
Changes in oral glucose-induced insulin secretion. An index of early-phase insulin secretion (ΔI0–30 min/ΔG0–30 min) was calculated as described by Abdul-Ghani et al. (1). A: the decrease in the eucaloric diet group (EX) was not significant [preintervention (pre) vs. postintervention (post): P > 0.05, n = 10]. B: following the 12-wk lifestyle intervention, insulin secretion was significantly reduced in the hypocaloric diet group (EX-HYPO; pre vs. post: *P < 0.05, n = 9). Data are presented as means ± SE.
GIP and PYY3–36.
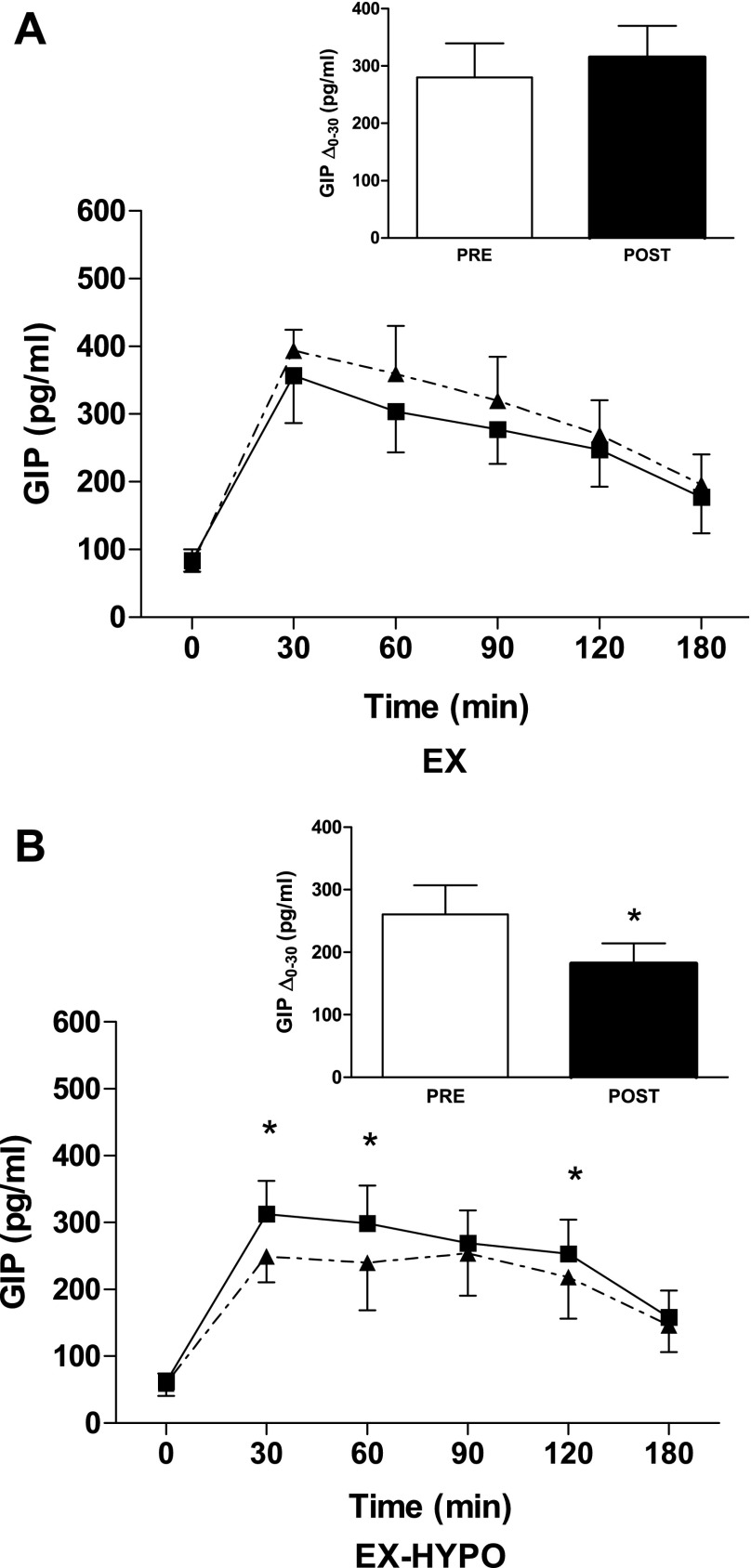
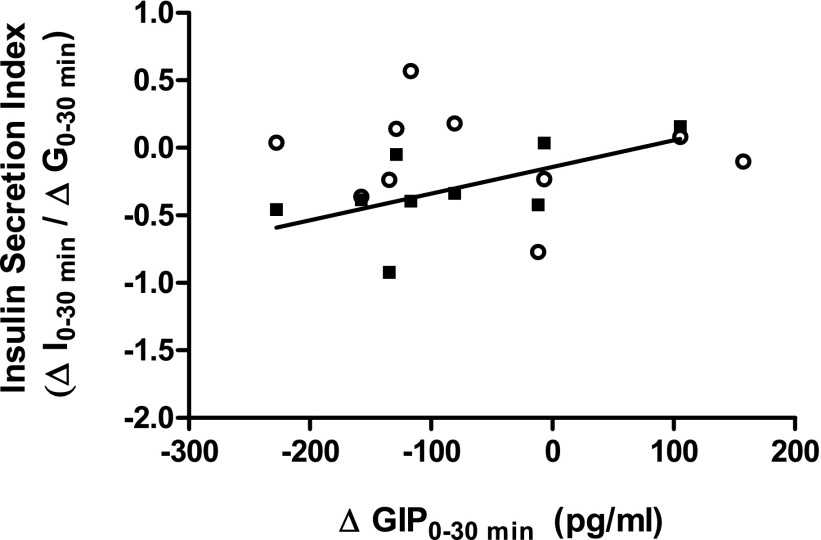
After the intervention, fasting GIP concentrations were unchanged in both groups (Table 2 and Fig. 2, A and B). However, the GIP response to glucose was significantly reduced in the EX-HYPO group compared with baseline and with the EX group (P = 0.04 and P = 0.007, respectively; Fig. 2B). Furthermore, the immediate GIP secretory response as determined by the change from time 0 to time 30 min was reduced in EX-HYPO compared with prestudy (P = 0.04; Fig. 2B, inset) and EX poststudy (P = 0.06). There was no change in the GIP response to glucose or secretion following EX (Fig. 2, A and A, inset, and Table 2). In addition, there was a significant correlation between the percent change in insulin and GIP after EX-HYPO (r = 0.85, P = 0.002; Fig. 3).
Fig. 2.
Plasma glucose-dependent insulinotropic polypeptide (GIP) responses to a 75-g oral glucose challenge. The GIP response to a 75-g oral glucose stimulus was measured before and after the EX (A) and EX-HYPO (B) interventions. The solid lines represent prestudy GIP responses to the oral glucose tolerance test (0–180 min), whereas dashed lines indicate poststudy responses. There was a significant decrease in the GIP response at t = 30, 60, and 120 min in the EX-HYPO group only; *P < 0.05. A and B, insets: immediate GIP secretory response to glucose ingestion, calculated as the change from t = 0 to 30 min (Δ0–30 min). Bars (white, pre; black, post) represent means ± SE. The GIP response was unchanged in the EX group (A, inset; pre vs. post: P > 0.05, n = 9), whereas there was a significant decrease in the EX-HYPO group (B, inset; pre vs. post: P = 0.04, n = 8).
Fig. 3.
Relationship between insulin secretion and the GIP response to oral glucose. The relationship between the change in glucose-induced insulin secretion (ΔI0–30 min/ΔG0–30 min) and the change in plasma GIP (ΔGIP0–30 min, pg/ml) in response to glucose following the interventions was assessed using bivariate correlation analyses. ○EX group; ▪EX-HYPO group. The correlation between the 2 variables was significant (r = 0.69, P = 0.05).
Fasting plasma PYY3–36 concentrations also remained unchanged by either intervention. However, the immediate PYY3–36 secretory response to glucose (Δ0–30 min, pg/ml) was significantly increased in both EX (pre: 12.5 ± 3.2, post: 25.9 ± 5.7; P = 0.01) and EX-HYPO (pre: 18.5 ± 4.3, post: 27.8 ± 5.9; P = 0.03) postintervention compared with prestudy. All of the PYY3–36 responses after glucose ingestion trended toward an increase in the EX-HYPO group (P = 0.05) but not in the EX group.
DISCUSSION
The effect of exercise on gut peptides, the gut peptide response to nutrient stimulation, and insulin secretion, especially in insulin-resistant conditions, has not been studied extensively. Our results show, for the first time, that an exercise/diet lifestyle intervention reduces plasma GIP and increases the PYY3–36 response following glucose stimulation in older, obese, insulin-resistant adults. This study also shows that the change in glucose-stimulated insulin secretion was related to the change in glucose-induced GIP response after the respective interventions. These data suggest that physical activity and moderate caloric restriction reduce insulin secretion via attenuation of a GIP-related incretin effect following nutrient stimulation, and this leads to improved glucose tolerance in a population that is at risk for the development of type 2 diabetes.
Previous studies in humans and animals have shown that exercise training by itself generally improves β-cell sensitivity and reduces the insulin response to glucose (16, 22, 30, 36). However, these findings are not universal, and a recent study in young subjects with type 2 diabetes reported no change in glucose-stimulated insulin secretion following exercise training (10). Insulin secretion has also been reported to increase after exercise training in subjects with type 2 diabetes (13). These different responses may be related to the effect of the exercise intervention on weight loss. The cited studies that did not find a reduced insulin response following exercise training also reported that subjects lost little if any body weight. In the present study, weight loss in the EX-HYPO group was substantially greater than EX alone, and greater changes in the glucose-stimulated insulin response and insulin secretion were clearly evident.
In the present study, the GIP response to glucose was reduced after the EX-HYPO intervention but was unchanged after EX alone. We had previously observed an attenuation of the GIP and insulin response to glucose ingestion after an acute bout of exercise in young trained athletes (34), and Blom et al. (6) reported a similar response in young untrained men. When combined with data from Krotkiewski et al. (23), who reported a reduced GIP response to exercise training in severely obese women, we were expecting a decrease in glucose-stimulated GIP after exercise training. However, all of the data supporting a decrease in the GIP response to glucose have been generated in younger subjects; it is likely that older, impaired, glucose-tolerant subjects may need a bigger stimulus to modify the K cell response and alter GIP release. Based on results from the EX-HYPO trial, it would seem that the greater weight loss that was accomplished by exercise and diet together may have provided enough of a stimulus to elicit this response. The observation that changes in the glucose-stimulated GIP response and changes in insulin secretion were correlated after the intervention lends support to the known incretin effect of GIP on insulin secretion (4, 15). Thus, it may be that the EX-HYPO intervention induces cellular changes in the β-cell and the K cell, which in turn helps to restore normal incretin function. The identification of the factors that mediate this effect and its related mechanism could not be accomplished in the current study but represent an area of emerging interest. Collectively, these observations lead us to suggest that weight loss, which can be more effectively achieved by a combination of exercise and diet, is an important determinant of improved β-cell function, improved K cell function, and possibly the cross-talk between these cells that allows GIP to help control insulin release in response to nutrient stimulation.
To date, little is known about the effects of exercise training alone, or calorie restriction in conjunction with exercise, on fasting or nutrient-stimulated PYY3–36 responses (17, 32, 37). Some studies have reported a short-lived increase in PYY3–36 levels during an acute exercise bout (25, 41), but it was shown recently that the PYY3–36 response to meal ingestion is elevated for up to 8 h after an acute bout of aerobic exercise (8). The present study is the first to address the effects of exercise/diet interventions on PYY3–36 levels in humans. In both EX and EX-HYPO there was an increase in the immediate PYY3–36 (Δ0–30 min) response to glucose, suggesting an improvement in PYY3–36 sensitivity following exercise training. Exercise has been reported to have an anorexic effect (21), and PYY3–36 is an anorexic hormone that inhibits food intake via hypothalamic signaling (5). Our results demonstrating an increase in glucose-stimulated PYY3–36 release, taken together with previous reports of an increase in PYY3–36 levels during exercise, suggest a possible mechanism whereby exercise can reduce food intake via increased sensitivity to satiety cues, ultimately leading to reductions in body weight.
We recognize that there are aspects of the current study design that limit the conclusiveness of our findings. The absence of a diet-only group limits our ability to distinguish between the effects of diet-induced responses vs. exercise/diet or exercise alone. Now that we have uncovered an exercise/diet effect on GIP, we will move forward with additional groups in future study designs. Furthermore, due to limited sample volume and lack of appropriate inhibitor, we did not measure the other major incretin hormone, GLP-1. We recognize the importance of GLP-1 in glucose-stimulated insulin secretion and in the regulation of glucose homeostasis (27, 33) and realize that alterations in GLP-1 levels may contribute to the changes we observed in insulin secretion and improvements in insulin resistance.
Data from this study provide evidence that physical activity and caloric restriction can improve gut peptide release, glucose-stimulated insulin secretion, and glucose tolerance, all of which are imperative in the prevention of progression from impaired glucose tolerance to type 2 diabetes. We suggest that GIP may play an important role in mediating the glucose-stimulated insulin response and that combined exercise/diet interventions provide an effective means to upregulate gut peptide function and prevent β-cell dysfunction in older, obese, insulin-resistant adults.
GRANTS
This research was supported by National Institutes of Health Grants AG-12834, RR-10732, RR-00080, RR-018390, and T32-DK-007319 and a grant from the Diabetes Association of Greater Cleveland (467-R-01).
Acknowledgments
We thank the nursing/dietary staff of the General Clinical Research Center and the research participants for their cooperation and commitment.
REFERENCES
- 1.Abdul-Ghani MA, Williams K, DeFronzo R, Stern M. Risk of progression to type 2 diabetes based on relationship between postload plasma glucose and fasting plasma glucose. Diabetes Care 29: 1613–1618, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Althage MC, Ford EL, Wang S, Tso P, Polonsky KS, Wice BM. Targeted ablation of glucose-dependent insulinotropic polypeptide-producing cells in transgenic mice reduces obesity and insulin resistance induced by a high fat diet. J Biol Chem 283: 18365–18376, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 32, Suppl 1: S62–S67, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen DK, Elahi D, Brown JC, Tobin JD, Andres R. Oral glucose augmentation of insulin secretion. Interactions of gastric inhibitory polypeptide with ambient glucose and insulin levels. J Clin Invest 62: 152–161, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batterham RL, Bloom SR. The gut hormone peptide YY regulates appetite. Ann NY Acad Sci 994: 162–168, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Blom PC, Høstmark AT, Flaten O, Hermansen L. Modification by exercise of the plasma gastric inhibitory polypeptide response to glucose ingestion in young men. Acta Physiol Scand 123: 367–368, 1985. [DOI] [PubMed] [Google Scholar]
- 7.Bourey RE, Kohrt WM, Kirwan JP, Staten MA, King DS, Holloszy JO. Relationship between glucose tolerance and glucose-stimulated insulin response in 65-year olds. J Gerontol 48: M122–M127, 1993. [DOI] [PubMed] [Google Scholar]
- 8.Broom DR, Batterham RL, King JA, Stensel DJ. Influence of resistance and aerobic exercise on hunger, circulating levels of acylated ghrelin, and peptide YY in healthy males. Am J Physiol Regul Integr Comp Physiol 296: R29–R35, 2009. [DOI] [PubMed] [Google Scholar]
- 9.Brubaker PL, Drucker DJ. Structure-function of the glucagon receptor family of G protein-coupled receptors: the glucagon, GIP, GLP-1, and GLP-2 receptors. Receptors Channels 8: 179–188, 2002. [PubMed] [Google Scholar]
- 10.Burns N, Finucane FM, Hatunic M, Gilman M, Murphy M, Gasparro D, Mari A, Gastaldelli A, Nolan JJ. Early-onset type 2 diabetes in obese white subjects is characterised by a marked defect in beta cell insulin secretion, severe insulin resistance and a lack of response to aerobic exercise training. Diabetologia 50: 1500–1508, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Cataland S, Crockett SE, Brown JC, Mazzaferri EL. Gastric inhibitory polypeptide (GIP) stimulation by oral glucose in man. J Clin Endocrinol Metab 39: 223–228, 1974. [DOI] [PubMed] [Google Scholar]
- 12.De Luis DA, Aller R, Izaola O, Gonzalez Sagrado M, Conde R, Perez Castrillon JL. Effects of lifestyle modification on adipocytokine levels in obese patients. Eur Rev Med Pharmacol Sci 12: 33–39, 2008. [PubMed] [Google Scholar]
- 13.Dela F, von Linstow ME, Mikines KJ, Galbo H. Physical training may enhance β-cell function in type 2 diabetes. Am J Physiol Endocrinol Metab 287: E1024–E1031, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Drucker DJ The role of gut hormones in glucose homeostasis. J Clin Invest 117: 24–32, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elahi D, Andersen DK, Muller DC, Tobin JD, Brown JC, Andres R. The enteric enhancement of glucose-stimulated insulin release. The role of GIP in aging, obesity, and non-insulin-dependent diabetes mellitus. Diabetes 33: 950–957, 1984. [DOI] [PubMed] [Google Scholar]
- 16.Farrell PA, Caston AL, Rodd D, Engdahl J. Effect of training on insulin secretion from single pancreatic beta cells. Med Sci Sports Exerc 24: 426–433, 1992. [PubMed] [Google Scholar]
- 17.Hanusch-Enserer U, Ghatei MA, Cauza E, Bloom SR, Prager R, Roden M. Relation of fasting plasma peptide YY to glucose metabolism and cardiovascular risk factors after restrictive bariatric surgery. Wien Klin Wochenschr 119: 291–296, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Jung SH, Park HS, Kim KS, Choi WH, Ahn CW, Kim BT, Kim SM, Lee SY, Ahn SM, Kim YK, Kim HJ, Kim DJ, Lee KW. Effect of weight loss on some serum cytokines in human obesity: increase in IL-10 after weight loss. J Nutr Biochem 19: 371–375, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Kahle EB, O'Dorisio TM, Walker RB, Eisenman PA, Reiser S, Cataland S, Zipf WB. Exercise adaptation responses for gastric inhibitory polypeptide (GIP) and insulin in obese children. Possible extra-pancreatic effects. Diabetes 35: 579–582, 1986. [DOI] [PubMed] [Google Scholar]
- 20.King DS, Staten MA, Kohrt WM, Dalsky GP, Elahi D, Holloszy JO. Insulin secretory capacity in endurance-trained and untrained young men. Am J Physiol Endocrinol Metab 259: E155–E161, 1990. [DOI] [PubMed] [Google Scholar]
- 21.King NA, Burley VJ, Blundell JE. Exercise-induced suppression of appetite: effects on food intake and implications for energy balance. Eur J Clin Nutr 48: 715–724, 1994. [PubMed] [Google Scholar]
- 22.Kirwan JP, Kohrt WM, Wojta DM, Bourey RE, Holloszy JO. Endurance exercise training reduces glucose-stimulated insulin levels in 60- to 70-year-old men and women. J Gerontol 48: M84–M90, 1993. [DOI] [PubMed] [Google Scholar]
- 23.Krotkiewski M, Björntorp P, Holm G, Marks V, Morgan L, Smith U, Feurle GE. Effects of physical training on insulin, connecting peptide (C-peptide), gastric inhibitory polypeptide (GIP) and pancreatic polypeptide (PP) levels in obese subjects. Int J Obes 8: 193–199, 1984. [PubMed] [Google Scholar]
- 24.Laferrere B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, Kovack B, Bawa B, Koshy N, Lee H, Yapp K, Olivan B. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 93: 2479–2485, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martins C, Morgan LM, Bloom SR, Robertson MD. Effects of exercise on gut peptides, energy intake and appetite. J Endocrinol 193: 251–258, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Matsuda M, DeFronzo R. Insulin sensitivity indices obtained from oral glucose tolerance testing. Diabetes Care 22: 1462–1470, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Meier JJ, Nauck MA. Glucagon-like peptide 1 (GLP-1) in biology and pathology. Diabetes Metab Res Rev 21: 91–117, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Meneilly GS, Ryan AS, Minaker KL, Elahi D. The effect of age and glycemic level on the response of the beta-cell to glucose-dependent insulinotropic polypeptide and peripheral tissue sensitivity to endogenously released insulin. J Clin Endocrinol Metab 83: 2925–2932, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Meneilly GS, Ryan AS, Veldhuis JD, Elahi D. Increased disorderliness of basal insulin release, attenuated insulin secretory burst mass, and reduced ultradian rhythmicity of insulin secretion in older individuals. J Clin Endocrinol Metab 82: 4088–4093, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Mikines KJ, Sonne B, Tronier B, Galbo H. Effects of training and detraining on dose-response relationship between glucose and insulin secretion. Am J Physiol Endocrinol Metab 256: E588–E596, 1989. [DOI] [PubMed] [Google Scholar]
- 31.Miyawaki K, Yamada Y, Ban N, Ihara Y, Tsukiyama K, Zhou H, Fujimoto S, Oku A, Tsuda K, Toyokuni S, Hiai H, Mizunoya W, Fushiki T, Holst JJ, Makino M, Tashita A, Kobara Y, Tsubamoto Y, Jinnouchi T, Jomori T, Seino Y. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med 8: 738–742, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Morinigo R, Moize V, Musri M, Lacy AM, Navarro S, Marin JL, Delgado S, Casamitjana R, Vidal J. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab 91: 1735–1740, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7–36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 36: 741–744, 1993. [DOI] [PubMed] [Google Scholar]
- 34.O'Connor AM, Pola S, Ward BM, Fillmore D, Buchanan KD, Kirwan JP. The gastroenteroinsular response to glucose ingestion during postexercise recovery. Am J Physiol Endocrinol Metab 290: E1155–E1161, 2006. [DOI] [PubMed] [Google Scholar]
- 35.O'Leary VB, Marchetti CM, Krishnan RK, Stetzer BP, Gonzalez F, Kirwan JP. Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J Appl Physiol 100: 1584–1589, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pratley RE, Hagberg JM, Dengel DR, Rogus EM, Muller DC, Goldberg AP. Aerobic exercise training-induced reductions in abdominal fat and glucose-stimulated insulin responses in middle-aged and older men. J Am Geriatr Soc 48: 1055–1061, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Reinehr T, Roth CL, Schernthaner GH, Kopp HP, Kriwanek S, Schernthaner G. Peptide YY and glucagon-like peptide-1 in morbidly obese patients before and after surgically induced weight loss. Obes Surg 17: 1571–1577, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Ryan AS, Pratley RE, Goldberg AP, Elahi D. Resistive training increases insulin action in postmenopausal women. J Gerontol A Biol Sci Med Sci 51: M199–M205, 1996. [DOI] [PubMed] [Google Scholar]
- 39.Salera M, Giacomoni P, Pironi L, Cornia G, Capelli M, Marini A, Benfenati F, Miglioli M, Barbara L. Gastric inhibitory polypeptide release after oral glucose: relationship to glucose intolerance, diabetes mellitus, and obesity. J Clin Endocrinol Metab 55: 329–336, 1982. [DOI] [PubMed] [Google Scholar]
- 40.Solomon TP, Sistrun SN, Krishnan RK, Del Aguila LF, Marchetti CM, O'Carroll SM, O'Leary VB, Kirwan JP. Exercise and diet enhance fat oxidation and reduce insulin resistance in older obese adults. J Appl Physiol 104: 1313–1319, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ueda SY, Yoshikawa T, Katsura Y, Usui T, Nakao H, Fujimoto S. Changes in gut hormone levels and negative energy balance during aerobic exercise in obese young males. J Endocrinol 201: 151–159, 2009. [DOI] [PubMed] [Google Scholar]
- 42.Verdich C, Toubro S, Buemann B, Lysgård Madsen J, Juul Holst J, Astrup A. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety—effect of obesity and weight reduction. Int J Obes Relat Metab Disord 25: 1206–1214, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Vincent RP, le Roux CW. Changes in gut hormones after bariatric surgery. Clin Endocrinol (Oxf) 69: 173–179, 2008. [DOI] [PubMed] [Google Scholar]
- 44.Yamada C, Yamada Y, Tsukiyama K, Yamada K, Yamane S, Harada N, Miyawaki K, Seino Y, Inagaki N. Genetic inactivation of GIP signaling reverses aging-associated insulin resistance through body composition changes. Biochem Biophys Res Commun 364: 175–180, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Zwirska-Korczala K, Konturek SJ, Sodowski M, Wylezol M, Kuka D, Sowa P, Adamczyk-Sowa M, Kukla M, Berdowska A, Rehfeld JF, Bielanski W, Brzozowski T. Basal and postprandial plasma levels of PYY, ghrelin, cholecystokinin, gastrin and insulin in women with moderate and morbid obesity and metabolic syndrome. J Physiol Pharmacol 58, Suppl 1: 13–35, 2007. [PubMed] [Google Scholar]