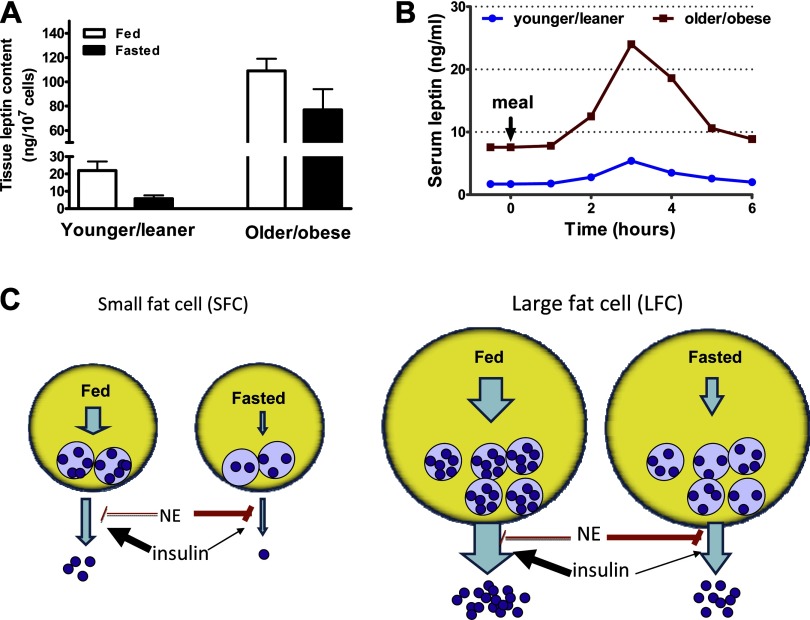
Fig. 2.
Influence of obesity (fat cell size, FCS) and meals on leptin storage and secretion. Large fat cells (LFC) from obese animals have larger stores of intracellular leptin compared with small fat cells (SFC) from lean controls. A: leptin content of younger/leaner (6–7 wk old, FCS = 0.05 μg lipid/cell) and older/obese (12–14 wk old, FCS = 0.17 μg lipid/cell) rat adipocytes reported in Refs. 38 and 40. (Note these were assayed at the same time so the values can be compared reliably.) B: data adapted from published papers (44, 47, 86) to illustrate that obesity is associated with higher fasting leptin levels and higher meal-induced leptin excursions. We hypothesize that this occurs, at least in part, as a result of increased secretion of preformed leptin from the LFC of the obese. C: in both SFC and LFC, the secretion of preformed leptin is modulated by insulin and catecholamines such as norepinephrine (NE) (38, 65). Under fasting conditions, in both large and small fat cells, the rate of secretion from preformed leptin stores is restrained by the increased sympathetic input to adipose tissue and higher NE and low insulin. After a meal, the rise in insulin and decrease in NE stimulates leptin secretion from preformed intracellular stores. Although drawn as one pool for simplicity, it is possible that leptin is stored in one or more as yet undefined endoplasmic reticulum and Golgi compartments and that obesity, as well as fasting and feeding, affects this distribution. Similarly, the number of vesicles, in addition to the leptin content per vesicle, may also be regulated as a function of nutritional status.