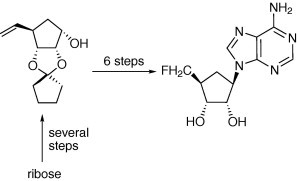
Graphical abstract
Keywords: Carbocyclic nucleoside, Grubbs reaction, Fluorinated cyclopentyl derivative, Mitsunobu coupling
Abstract
5′-Fluoro-5′-deoxyaristeromycin (2) has been prepared via a Mitsunobu coupling of (1S,2S,3R,4S)-2,3-(cyclopentylidenedioxy)-4-fluoromethylcyclopentan-1-ol with N6-bis-boc protected adenine. This procedure is adaptable to preparing a number of 5′-fluoro-5′-deoxycarbocyclic nucleoside analogs with diversity in the heterocyclic base. Antiviral analysis found promising activity for 2 toward measles but no other viruses. No cytotoxicity was observed for 2.
Aristeromycin (1) owes its diverse biological properties to (1) formation of its 5′-mono-, di-, and triphosphate nucleotides and (2) inhibition of biomethylations that occur with S-adenosylmethionine (AdoMet) as cofactor and S-adenosylhomocysteine as a product biofeedback methylation control element.1 In an effort to investigate aristeromycin derivatives that are participants in only one of those pathways we, for some time, have sought aristeromycin analogs where C-5′ phosphorylation is not likely2, 3, 4 or not possible.5 For this collection, 5′-fluoro-5′-deoxyaristeromycin (2) presents a relevant target owing to the well-documented beneficial biological consequences of a fluoro-for-hydroxyl exchange, which, for the purposes here, also precludes the traditional hydroxyl reactions (such as, phosphate esterification).6 Additionally, 2 represents the carbocyclic analog of 5′-fluoro-5′-deoxyadenosine, which has demonstrated a broad range of biological properties.7 Our preliminary results in this regard are reported (Fig. 1 ).
Figure 1.
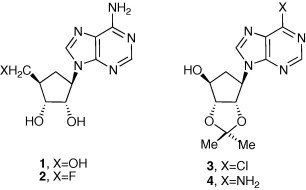
Our original plan to 2 was to carry out a direct fluorination at the C-5′ center of the known aristeromycin precursor 3 or 4.8 In that direction we found it synthetically more practical to prepare the unknown cyclopentyl protected derivative 5 employing a series of new cyclopentyl derivatives (via Scheme 1 ). However, various attempts at converting 5–6 (e.g., using DAST shown as example in Scheme 1) were unsuccessful.
Scheme 1.
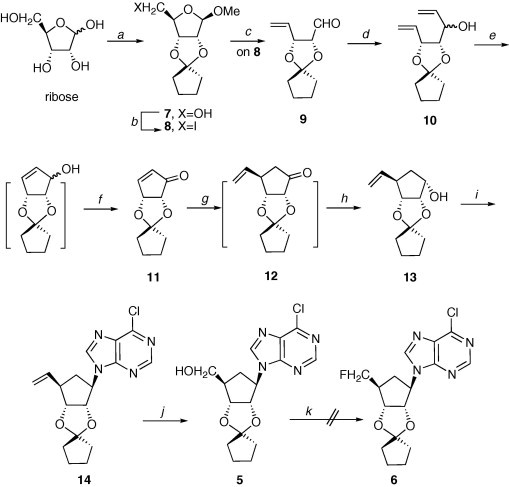
Reagents and conditions: (a) cyclopentanone, MeOH, (MeO)3CH, 5% p-TSA, reflux, 85%; (b) I2, imidazole, TPP, tolune/MeCN, reflux, 80%; (c) n-BuLi, −78 °C, ether, 80%; (d) CH2CHMgBr, dry Et2O, −78 °C to rt, 80%; (e) 1st gen. Grubbs cat. 1% mol, reflux, CH2Cl2; (f) SO3–pyrridine, DIPEA, DMSO, CH2Cl2, 0 °C, 70% two steps; (g) CH2CHMgBr, CuBr–Me2S, TMSCl, THF, −78 °C to rt; (h) LiAlH4, THF, 62% two steps; (i) 6-Cl–purine, DIAD, THF, 60 °C, 2 days, 60%; (j) NaIO4, OsO4, MeOH/H2O, then NaBH4, MeOH, 68%; (k) one example, DAST, CH2Cl2, reflux.
Attention then turned to creating a fluoro-bearing cyclopentyl unit for coupling with an appropriately functionalized purine base. Scheme 2 presents the steps employed to the requisite precursor 15. To circumvent the interference by the C-6 amino substituent of adenine under the Mitsunobu coupling conditions,9 N 6-bis-boc protected adenine was employed with 15 in the presence of diiso’propyl azodicarboxylate to yield 19. Acidic deprotection of 19 led to the desired 2.10
Scheme 2.
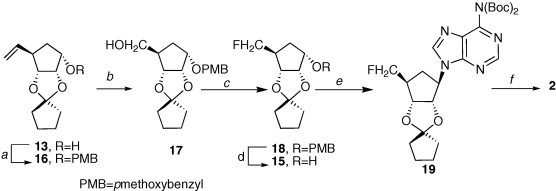
Reagents and conditions: (a) NaH, PMBCl, DMF, 0 °C, 81%; (b) NaIO4, OsO4, MeOH/H2O, then NaBH4, MeOH, 53%; (c) DAST, pyridine, CH2Cl2, 76%; (d) DDQ, CH2Cl2/H2O, 60%; (e) DIAD, TPP, Ad(Boc)2; (f) 3 N HCl/MeOH, 58% for two steps.
To gain a glimpse into the biological potential of 2, it was subjected to antiviral analysis versus herpes simplex-1, herpes simplex-2, herpes simplex-1 (TK−), vaccinia, cowpox, vesicular stomatitis, coxsackie B4, respiratory syncytial, parainfluenza 3, reovirus-1, Sindbis, Punta Toro, rhinovirus, adenovirus, hepatitis C, and West Nile, and feline coronavirus.11 No activity was found for these viruses. However, effects toward measles (MO6) were observed (EC50 2.8 μM, neutral red assay; EC50 13 μM, visual assay). As a member of the paramyxoviridae family of viruses it might be expected that some activity would be seen with an aristeromycin analog since this viral family is susceptible to inhibition of S-adenosylhomocysteine metabolism.12 It is surprising, however, that the other paramyxo representatives (respiratory syncytial and parainfluenza) and none of the other viruses (e.g., vaccinia, cowpox, vesicular stomatitis, and reo) that are vulnerable to such interference were not affected by 2.12 Perhaps, another mechanism is selectively operative for 2 toward measles.13 No cytotoxicity arose in the cell lines used for the antiviral assays.14
Acknowledgments
This research has been supported by funds from the Department of Health and Human Services (Al 56540), which is appreciated. We are also indebted to the following individuals for providing the antiviral data11 following their standard protocols: Drs. Erik De Clercq and Jan Balzarini, the Rega Institute, Leuven Belgium; Drs. Earl Kern and Mark Prichard, University of Alabama at Birmingham, Birmingham, AL; Drs. Robert Sidwell and Don Smee, Utah State University, Logan, UT; and, Dr. Brent Korba, Georgetown, University, Rockville, MD.
References and notes
- 1.Wolfe M.S., Borchardt R.T. J. Med. Chem. 1991;34:1521. doi: 10.1021/jm00109a001. [DOI] [PubMed] [Google Scholar]
- 2.Roy A., Serbessa T., Schneller S.W. Bioorg. Med. Chem. 2006;14:4980. doi: 10.1016/j.bmc.2006.03.011. For a recent 5′-noraristeromycin leading reference, see. [DOI] [PubMed] [Google Scholar]
- 3.Ye W., Schneller S.W. J. Org. Chem. 2006;71:8641. doi: 10.1021/jo0612170. [DOI] [PubMed] [Google Scholar]
- 4.Yang M., Schneller S.W. Bioorg. Med. Chem. Lett. 2005;15:149. doi: 10.1016/j.bmcl.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Siddiqi S.M., Schneller S.W. Nucleosides Nucleotides. 1993;12:185. [Google Scholar]
- 6.Kirk K.L. J. Fluorine Chem. 2006;127:1013. For a leading reference, see. [Google Scholar]
- 7.Ashton T.D., Scammells P.J. Bioorg. Med. Chem. Lett. 2005;15:3361. doi: 10.1016/j.bmcl.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 8.Yang M., Ye W., Schneller S.W. J. Org. Chem. 2004;69:3993. doi: 10.1021/jo040119g. [DOI] [PubMed] [Google Scholar]
- 9.(a) Mitsunobu O. Synthesis. 1981:1. [Google Scholar]; (b) Hughes D.L. Org. Prep. Proced. Int. 1996;28:127. [Google Scholar]
- 10.Selected data for 2: white solid; mp 168–169 °C. 1H NMR (400 MHz, DMSO), δ 8.19 (s, 1H), 8.12 (s, 1H), 7.20 (s, 2H), 5.06 (d, J = 6.0 Hz, 1H), 4.91 (d, J = 4.0 Hz, 1H), 4.70 (m, 1H), 4.58 (m, 1H), 4.45 (m, 1H), 4.35 (m, 1H), 3.89 (m, 1H), 2.27 (m, 2H), 1.80 (m, 1H). 13C NMR (100 MHz, DMSO) δ 156.01, 152.11, 149.68, 140.17, 119.35, 84.55 (d, J = 165 Hz), 74.26, 70.76 (d, J = 5 Hz), 59.13, 43.40 (d, J = 18 Hz), 27.86 (d, J = 6 Hz). Calcd mass for C11H14FN5O2: 267.1132. Found: 267.1131.
- 11.(a) Rajappan V.P., Schneller S.W., Williams S.L., Kern E.R. Bioorg. Med. Chem. 2002;10:883;. doi: 10.1016/s0968-0896(01)00344-3. For leading references for the antiviral and cytotoxicity procedures used, see. [DOI] [PubMed] [Google Scholar]; (b) Siddiqi S.M., Chen X., Schneller S.W., Ikeda S., Snoeck R., Andrei G., Balzarini J., De Clercq E. J. Med. Chem. 1994;37:551. doi: 10.1021/jm00030a014. [DOI] [PubMed] [Google Scholar]; (c) http://www.usu.edu/iar/Brochure/brochure.html (October 1, 2007).
- 12.De Clercq E. Nucleosides Nucleotides. 1998;17:625. doi: 10.1080/07328319808005205. [DOI] [PubMed] [Google Scholar]
- 13.Shuto S., Obara T., Toriya M., Hosoya M., Snoeck R., Andrei G., Balzarini J., De Clercq E. J. Med. Chem. 1992;35:324. doi: 10.1021/jm00080a018. The 5′-fluoro-5′-deoxyneplanocin A analog of 2 also shows activity toward measles but it also had an effect on other viruses susceptible to inhibition of S-adenosylhomocysteine metabolism. [DOI] [PubMed] [Google Scholar]
- 14.Cell lines used for the antiviral assays: HEL, HeLa, Vero, CV-1, MA-104, A-549, MDCK, CRFK, HFF, Huh7ET.


