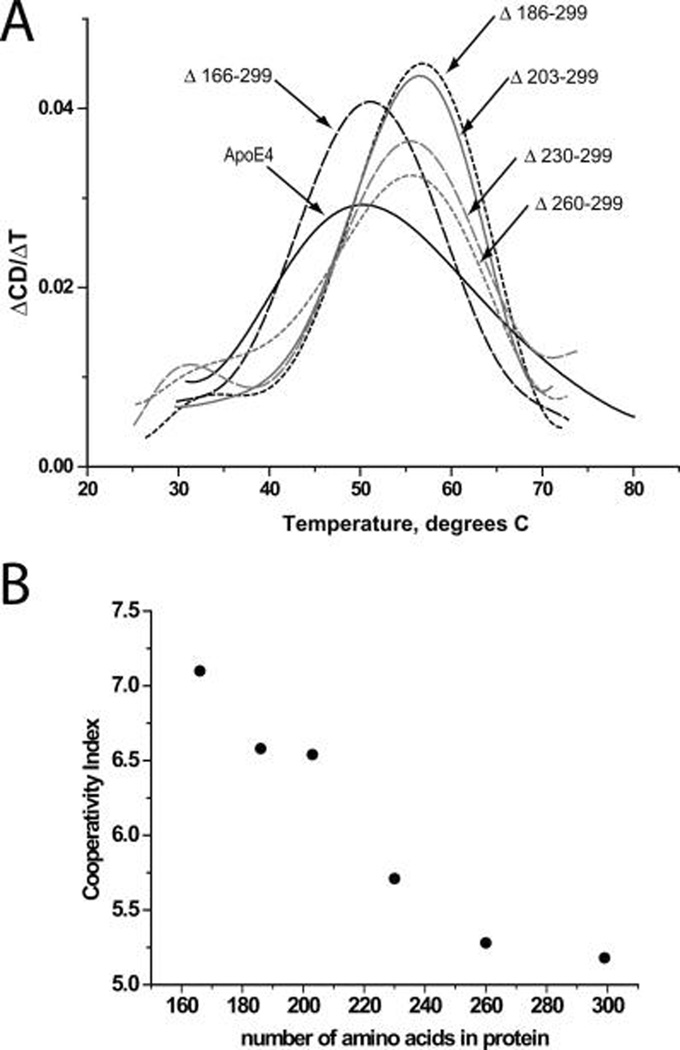
FIGURE 2.
(A) First-order derivative of thermal denaturation traces of wild-type apoE4 and C-terminal deletion mutants. Solid black line, wild-type apoE4; short-dash gray line, apoE4 Δ260–299; long-dash gray line, apoE4 Δ230–299; solid gray line, apoE4 Δ203–299; short-dash black line, apoE4 Δ186–299; long-dash black line, apoE4 Δ166–299. Wild-type apoE4 had a Tm of 50.2 °C and all deletion mutants with the exception of Δ166–299 exhibited elevated Tm values. (B) Plot of cooperativity index for thermal unfolding for wild-type and apoE4 mutants versus total number of amino acids in the mutant protein. Notice how the cooperativity index increases in almost a linear fashion as the C-terminus of apoE4 is truncated.