Abstract
The current study assessed neurotrophin protein levels in male and female rat brain tissues at four different ages ranging from postpuberty to senescence. In both sexes nerve growth factor (NGF) increased, and brain-derived neurotrophic factor (BDNF) decreased, from 4 to 24 months of age. Using a slightly older age for the young group, or a slightly younger age for the aged group, had profound effects on whether age effects were realized. There were no sex differences in the pattern of change in neurotrophin levels across age, and neurotrophin levels did not correlate with estrogen levels in females or estrogen or testosterone levels in males. The current findings suggest that profound changes in neurotrophin protein levels can occur within only a few months time, and that these changes influence whether age-related neurotrophin alterations are realized.
Survival and functional maintenance of cholinergic neurons are dependent upon neurotrophins, including nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) (Granholm, 2000; Levi-Montalcini, 1987; Woolf, 1991). Given recent evidence that neurotrophins are related to cognition in rodents, changes in neurotrophin levels may be a critical link in the cascade of biological alterations resulting in cognitive deterioration that occurs in aging and age-related neurodegenerative disorders (Kaisho, Ohta, Miyamato, & Igarashi, 1999; Mizuno, Yamada, Olariu, Nawa, & Nabeshima, 2000; Sugaya et al., 1998). Some studies show that patients with Alzheimer’s disease (AD) exhibit alterations in NGF and BDNF in various brain regions compared to age-matched controls, although such effects are not consistently reported (see Siegel & Chauhan, 2000, for review). Unfortunately, animal research has not clarified the direction of age-related neurotrophic alterations, or even whether changes do in fact occur. For example, compared to young rodents, aged rodents have exhibited both decreases (Nishizuka et al., 1991) or no change (Alberch, Perez-Navarro, Arenas, & Marsal, 1991; Crutcher & Weingartner, 1991; Katoh-Semba, Semba, Takeuchi, & Kato, 1998; Narisawa-Saito & Nawa, 1996; Scott, Liang, Weingartner, & Crutcher, 1994; Taglialatela, Robinson, Gegg, & Perez-Polo, 1997) in hippocampal NGF. Although strain differences in age-related neurotrophin changes may account for some of the discrepancy between rat studies, there are conflicting findings even when comparing studies using the same rat strain (Larkfors et al., 1988).
Differences in the age of respective young and old groups may account for some variability in the findings. Showing substantial variability, the age of the “aged” group has ranged from 18 to 33 months in different studies (Narisawa-Saito & Nawa, 1996; Yurek & Turner, 2001). The age of the young comparison group could also impact the young versus aged comparison, as underscored by research showing significant alterations in NGF and BDNF concentrations during early development (Katoh-Semba, 1997; Nishizuka et al., 1991). In some studies the age of the “young” group was 1 month (Katoh-Semba et al., 1998), 3 to 4 months (Nishizuka et al., 1991), 3 to 5 months (Bimonte, Nelson, & Granholm, 2002), or 4 to 5 months (Yurek & Turner, 2001), whereas others collapsed across 2 to 5 months olds (Scott et al., 1994). Thus, there is no general consensus as to how old “aged” or “ young” rats should be in aging studies. This leads us to question, how young is young, and how old is old, for neurotrophin changes to be realized?
Discordant findings between aging studies may also be related to the fact that some studies used males (e.g., Narisawa-Saito & Nawa, 1996; Yurek & Turner, 2001), whereas others used females (e.g., Scott et al., 1994). Still others did not specify the sex of subjects (Larkfors et al., 1988), or used both males and females yet did not include sex in the published analyses (Katoh-Semba et al., 1998). The importance of sex is highlighted by the reported sex differences in certain brain regions (e.g., the hippocampus) and biochemical systems (e.g., cholinergic and neurotrophic) known to degenerate during normal and disease-related aging (see Luine, Renner, & McEwen, 1986; Nishizuka et al., 1991, for examples). Hence, age-related changes in neurotrophin levels in males may not be generalizable to the age-related changes that occur in females.
The current study sought to determine whether the precise age at evaluation influences whether “age differences” are realized, and whether patterns of neurotrophin levels across the adult lifespan differ depending on sex. Hence, we assessed neurotrophin protein levels in male and female rats at several ages ranging from postpuberty to senescence. We focused on brain regions known to be influenced in their function by neurotrophins and that are affected in age-related neurodegenerative disorders that result in a profile of memory impairment. Serum gonadal hormone levels were also evaluated in order to examine whether such changes coincide and correlate with age-related alterations in growth factors.
METHODS
Subjects were male and female virgin Fischer 344 rats born and raised at Harlan Laboratories. The number of subjects in the female and male groups, respectively, were 8 and 9 in the 4-month group, 8 and 7 in the 7-month group, 7 and 6 in the 20-month group, and 5 and 6 in the 24-month group. After shipment to the Medical University of South Carolina, animals were pair housed with a like-age and -sex cagemate in an animal facility in a barrier environment, had exposure to food and water ad libitum, and were maintained on a 12-h light/dark cycle. Male and female rats were housed in the same room. All rats were sacrificed and brains were dissected during the same dissection session. Sacrifice was performed by one experimenter, and brain dissections by another experimenter who was blind to the age and sex of the animal. Animals were anesthetized and their brains rapidly removed and dissected. From the right hemisphere, the frontal cortex, entorhinal cortex, CA1/CA2 region of the hippocampus, and striatum were dissected and placed in pre-weighed microcentrifuge tubes in a −70° freezer until analysis. For each brain the frontal cortex was taken first from the dorsal aspect of the intact brain. Next, the brain was cut in the coronal plane using a razor blade to obtain access to each of the other three brain regions. For the frontal cortex, the most medial 1.5 to 2mm portion of the frontal cortex was dissected; for the CA1/CA2 region of the hippocampus, the dentate gyrus and the alveus were excluded; for the entorhinal cortex, the tissue was dissected from the same slice as the hippocampal sample, taking a 2- to 3-mm sample ventral to the hippocampus; and for the striatum, the dorsorostral striatum was dissected using corpus callosum as the dorsal and lateral border, and the anterior commissure as the ventral and caudal (crossing) border.
NGF and BDNF levels were assessed in each region using a commercially available assay kit from Promega. In brief, flat-bottom plates were coated with the corresponding capture antibody. The captured neurotrophin was bound by a second specific antibody, which was detected using a species-specific antibody conjugated to horseradish peroxidase. All unbound conjugates were removed by subsequent wash steps according to the standard Promega protocol. After incubation with chromagenic substrate, color change was measured in an enzyme-linked immunosorbent assay (ELISA) plate reader (Molecular Devices) at 450 nm. Using the Promega kits, NGF and BDNF can be quantified in the range of 7.8 to 500 pg/ml. For each assay kit, cross-reactivity with other trophic proteins is <2% to 3%.
At the time of brain dissection, trunk blood was obtained in order to ascertain circulating gonadal hormone levels. Testosterone concentrations in rat serum were measured in our laboratory, in duplicate, using commercially available ELISA kits (testosterone: DSL-10-4000; Diagnostic Systems Laboratories, Webster, TX). The assay procedure described in the enclosed protocol was followed. Estradiol hormone assays were performed by the Core Endocrinology Laboratory at Pennsylvania State University College of Medicine. Estradiol was determined in serum by radioimmunoassay following extraction with diethyl ether. Serum (2.4 ml) were extracted and the ether portion collected and evaporated. The sample was reconstituted in assay buffer and a competitive radioimmunoassay was performed using 125I estradiol with high specific activity and a highly specific antibody. Separation of bound from free estrogen was achieved with activated charcoal and the data reduction was performed with the use of a 5-point standard curve and purified estradiol standards. The functional sensitivity of the assay was 5 pg/ml.
The experiment was designed such that we could analyze the data in a factorial analysis of variance (ANOVA). This type of experimental design and analysis is especially useful when the interaction between groups is of particular interest. To assess changes in NGF and BDNF from one age to the next, consecutive ages were analyzed with age and sex as between factors. One of the main objectives of this study was to determine whether age-related changes in NGF and BDNF vary as a function of sex. Hence, although we evaluated the Sex × Age interaction, which was representative of sex differences in change across time, these effects were not significant so they are not described in Results. Thus, we report the main effect of age to represent changes from one age to the next, collapsed across sex. Because an additional interest was in sex differences at specific developmental time points, mean differences between the sexes were assessed and described at each age via t tests. Pearson r correlations were used to evaluate relationships between neurotrophins and gonadal hormone levels.
RESULTS
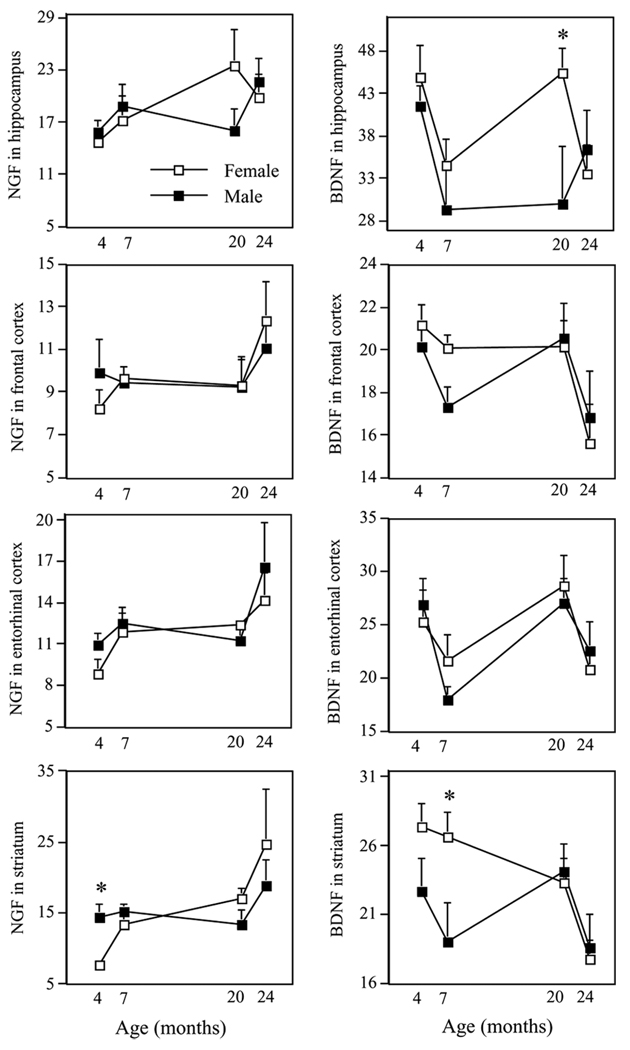
Table 1 shows all significant and marginally significant Age main effects for NGF and BDNF in each brain region. Figure 1 schematically represents mean ± SE NGF and BDNF levels for males and females for all evaluated brain regions at each age.
Table 1.
Significant or marginally significant p values for age effects for NGF and BDNF in each evaluated brain region
| Age comparison | |||||||
|---|---|---|---|---|---|---|---|
| Neurotrophin | Region | 4 vs. 7 | 7 vs. 20 | 20 vs. 24 | 4 vs. 20 | 7 vs. 24 | 4 vs. 24 |
| NGF | hippo | .05 | ns | ns | .09 | ns | .01 |
| frontal cortex | ns | ns | ns | ns | .06 | .09 | |
| ent cortex | .04 | ns | .09 | .05 | ns | .006 | |
| striatum | .08 | .08 | ns | .02 | .04 | .004 | |
| BDNF | hippo | .003 | ns | ns | ns | ns | .03 |
| frontal cortex | ns | ns | .02 | ns | ns | .004 | |
| ent cortex | .01 | .002 | .03 | ns | ns | ns | |
| striatum | ns | ns | .02 | ns | .06 | .01 | |
Note. hippo =hippocampus; ent =entorhinal; ns =not significant.
Figure 1.
Mean ± SE NGF and BDNF protein levels (pg/mg) in the hippocampus, frontal cortex, entorhinal cortex, and striatum for 4-, 7-, 20-, and 24-month-old male and female Fisher 344 rats. *p < .05, representing the sex difference at that particular developmental time point. See Table 1 for statistical values of the particular age comparisons.
Change in Neurotrophin Levels with Age
We found that hippocampal NGF levels increased from 4 to 7 months (age main effect: F(1, 27) = 4.21; p = .05), whereas levels did not change from 7 to 20, or 20 to 24, months of age. In contrast, hippocampal BDNF decreased from 4 to 7 months of age (age main effect: F(1, 28) = 10.37; p < .0050 and did not significantly change from 7 to 20, or 20 to 24, months of age.
NGF levels in the frontal cortex did not significantly change from 4 to 7, 7 to 20, or 20 to 24 months of age. Frontal cortex BDNF did not change from 4 to 7, or 7 to 20, months of age, but decreased from 20 to 24 months (F(1, 20) = 6.98; p < .05), with levels reaching their lowest point at 24 months.
The pattern of change across age for entorhinal cortex NGF and BDNF were in opposite directions. Indeed, entorhinal cortex NGF increased from 4 to 7 months (F(1, 28) = 4.65; p < .05), did not significantly change from 7 to 20 months, and marginally increased from 20 to 24 months (F(1, 20) = 3.17; p = .09). Conversely, BDNF levels in entorhinal cortex decreased from 4 to 7 months (F(1, 28) = 6.88; p < .05), increased from 7 to 20 months (F(1, 24) = 11.83; p < .005), and decreased from 20 to 24 months (F(1, 20) = 5.73; p < .05).
Striatal NGF levels increased from 4 to 7 months of age (F(1, 28) = 5.41; p < .05), and did not change from 7 to 20, or 20 to 24, months of age. Although BDNF levels in striatum did not significantly change from 4 to 7, or 20 to 24, months of age, they decreased from 20 to 24 months (F(1, 18) = 7.16; p < .05).
Specific Age Comparisons of Neurotrophin Levels
Many studies compare one group of young subjects to one group of aged subjects to evaluate “age differences” in neurotrophin levels. To assess whether the specific age of the young or aged group influences the magnitude of the age effect, we compared the different ages.
4 versus 24 months
The most profound age differences were seen in the comparison of 4-versus 24-month-old animals; the most extreme ages evaluated. Indeed, all but one evaluation showed significant or marginal age effects. Aged rats exhibited higher NGF levels in the hippocampus (F(1, 24) = 7.37; p < .05), frontal cortex (F(1, 24) = 3.23; p = .09), entorhinal cortex (F(1, 24) = 9.25; p < .01), and striatum (F(1, 24) = 10.05; p < .005). In contrast, aged rats showed lower levels of BDNF in the hippocampus (F(1, 24) = 5.12; p < .05), frontal cortex (F(1, 24) = 10.15; p < .005), and striatum (F(1, 22) = 8.01; p < .01).
4 versus 20 months
When “age differences” were analyzed by comparing rats at 4 versus 20 months of age, the effects were not as prominent as the comparison of 4-versus 24-month-old rats. NGF values were higher in 20-versus 4-month-olds in striatum (F(1, 26) = 6.39; p < .05), and somewhat higher in entorhinal cortex (F(1, 26) = 4.15; p = .05) and hippocampus (F(1, 25) = 3.13; p = .09). Although 4-month-old rats showed significantly higher BDNF levels in several regions as compared to 24-month-old rats, 4-month-old rats did not differ from 20-month-old rats for BDNF in any region (all p values > .15). This underscores the important changes that occur in BDNF levels from 20 to 24 months of age.
7 versus 24 months
Regarding the profound age effects noted in growth factors between 4- and 24-month-old animals (see Table 1), the majority of these comparisons were rendered null or marginal when 7-month-old animals were used as the young comparison group. Indeed, age effects for entorhinal cortex and hippocampal NGF were null when using 7-instead of 4-month-olds for the young group. Yet, compared to the 7-month-olds, the 24-month-old group did have significantly higher NGF levels in striatum (F(1, 22) = 4.66; p < .05), and marginally higher NGF levels in frontal cortex (F(1, 22) = 4.09; p = .06).
Compared to the 7-month-old group, BDNF levels were marginally lower in the 24-month-old group in the striatum (F(1,21) = 3.93; p = .06); whereas there were no differences in the hippocampus, frontal cortex, or entorhinal cortex. It is noteworthy that the large age effects seen in both hippocampal NGF and BDNF when comparing 4-versus 24-month-old rats were no longer significant when using the 7-month-old animals as the comparison young group.
Sex Differences in Neurotrophin Levels at Discrete Time Points
To evaluate sex differences in neurotrophins at different developmental timepoints, we compared mean neurotrophin values at each age. Compared to females, males had lower BDNF levels in the striatum at 7 months (F(1, 13) = 5.79; p < .05) and in the hippocampus at 20 months (F(1, 11) = 4.95; p < .05) of age. Males also had higher NGF levels in the striatum at 4 months of age (F(1, 15) = 9.84; p < .01).
Age-Related Changes in Gonadal Hormones
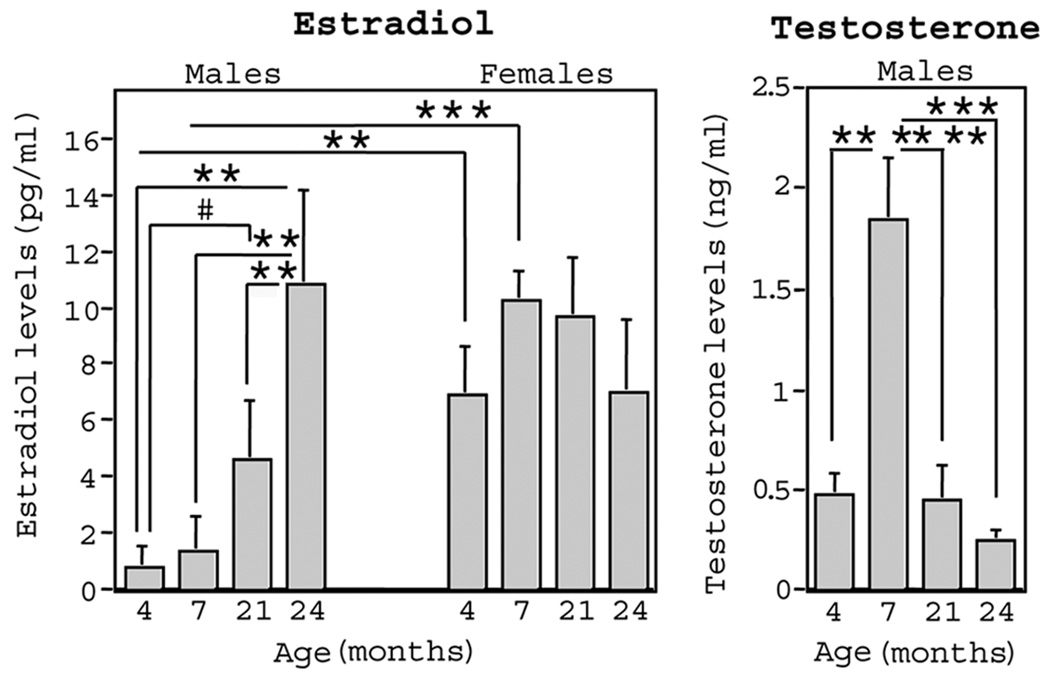
We wanted to evaluate whether age-related changes in growth factor levels correspond to age-related changes in gonadal hormone levels. Thus, we also evaluated serum levels of estradiol in female rats, and serum levels of estradiol and testosterone in male rats. Figure 2 shows mean ± SE levels of gonadal hormones in male and female rats at 4, 7, 20, and 24 months of age. There was a systematic increase in estradiol levels from 4 to 24 months of age in male rats. This resulted in a marginal increase by 20 months (t(11) = 2.09; p = .06), and a significant increase by 24 months (t(12) = 3.38; p < .01), as compared to 4-month-old male rats. In contrast, there was no change in estradiol levels in female rats across age. Sex difference analyses revealed that estradiol levels were higher in females versus males at 4 months (t(14) = 3.30; p < .01) and 7 months (t(13) = 5.50; p < .0005), but not at 20 and 24 months, of age. In male rats there was a substantial increase in testosterone from 4 to 7 months (t(13) =4.35; p < .001), and a decrease from 7 to 20 months (t(11) = 3.75; p < .005). Testosterone levels remained stable from 20 to 24 months of age.
Figure 2.
At the time of brain dissection, blood serum was collected to measure circulating gonadal hormone levels in 4-, 7-, 20-, and 24-month-old male and female rats (represented as mean ± SE). Estradiol was evaluated in both males and females, whereas testosterone was evaluated only in males. Estradiol levels increased with age in males, whereas no significant changes occurred in females. Testosterone increased from 4 to 7 months and decreased between 7 and 20 months in males. Correlation analyses showed that gonodal hormone levels did not correlate with neurotrophin protein levels for any group of animals.
Pearson r correlations were used to evaluate relationships between neurotrophin and gonadal hormone levels. Because there were significant age differences for hormones and growth factors, each age group within each sex was analyzed separately to prevent false significant correlations due to main effects of age. There were no significant correlations between neurotrophins and testosterone or estradiol in males, or neurotrophins and estradiol in females, at any age.
DISCUSSION
The most important finding in this study is that significant age effects depended on the specific age of the young and aged groups. It appears that even a few months of age can make a large difference in whether “aging effects” are observed for neurotrophin protein levels. For example, 24-month-old rats exhibited significantly more NGF and less BDNF protein in three brain regions as compared to 4-month-old rats. Yet, when we compared 24- to 7-month-old rats, just 3 months older than the youngest group examined, only one brain region showed a significant age effect for NGF, and no regions showed a significant age effect for BDNF. Similarly, 4 months in older rats (from 20 to 24 months of age) also makes a significant impact on the observation of age effects in neurotrophin levels. When 20-month-old rats were compared to 4-month-old rats, only one brain region for NGF and no regions for BDNF remained significantly different between “young” and “aged” rats. These findings likely explain why we did not find significant age-related changes in NGF and BDNF protein levels in previous work (Bimonte et al., 2002). Indeed, in that study we utilized female Fisher-344 rats that were 3 to 5 months of age for the young group, and 21 to 23 months of age for the aged group. In accordance with the current findings, we have also reported a significant elevation of hippocampal NGF protein levels in cognitively impaired male Fisher 344 rats at a later age, 24 to 25 months (Albeck et al., 1988). The results from the current study might also partially explain the wide variety of findings in studies evaluating “age alterations” in neurotrophins from different research groups that utilize different ages for the young versus aged groups (see the introductory section). In the current study, the exact age of the animals at the time of evaluation clearly affected the magnitude, and in some cases even the realization of, the age effect. Sex did not affect the pattern of change in neurotrophin protein levels with age, and estrogen levels in females and estrogen or testosterone levels in males did not correlate with neurotrophin protein levels at any age for any brain region.
In accordance with our observed age-associated increase in NGF protein levels in entorhinal and frontal cortex, others have shown an increase in frontal cortex NGF from 3 to 24 months in Sprague-Dawley rats (Hellweg et al., 1990). Still, many studies report a lack of change in neocortical NGF protein in Fischer 344 rats from 6 to 28 months of age (Larkfors et al., 1988), in Sprague-Dawley rats from 6 to 24 (Larkfors et al., 1988) or 6 to 36 (Alberch et al., 1991) months of age, nor in Fischer 344/Brown Norway F1 hybrid male rats from 3 to 30 months of age (Taglialatela et al., 1997). However, in many studies examining growth factors in neocortex, the particular cortical region (e.g., frontal, parietal, entorhinal) was not described. The specific neocortical region appears to be important when evaluating growth factor changes with age. The herein study found that there was a marginal increase from 4 to 24 months in frontal cortex for NGF, whereas there was a significant increase from 4 to 24 months for entorhinal cortex. We also showed a significant decrease from 4 to 24 months in frontal cortex for BDNF, but in contrast, no change for this same age comparison for entorhinal cortex. Hence, we report different age-related changes in growth factors depending on the specific cortical region. Within this framework, descriptive information including detail of the cortical region examined will be useful to aid in generalizability across studies.
We found changes in neurotrophin protein levels from 4 to 7 months of age for both neurotrophins examined. In the hippocampus, NGF marginally increased, and BDNF significantly decreased, from 4 to 7 months of age. For the entorhinal cortex, the pattern was similar, with NGF significantly increasing and BDNF significantly decreasing from 4 to 7 months of age. In contrast, there were no significant changes in frontal cortex and striatum for either neurotrophin. The large increase in serum testosterone levels that we observed from 4 to 7 months of age in males may be relevant to the observed neurotrophin changes in the male rats. It may also be of relevance that the number of neurons in some regions of the hippocampal formation substantially increase through at least 365 days of age (Bayer, Yackel, & Puri, 1982). Because there is increasing evidence that neurotrophins can induce formation and/or promote maturation of excitatory and inhibitory synapses in the hippocampus, it follows that neurotrophins may be involved in development or maturation of the hippocampus and intimately related areas such as the entorhinal cortex (Vicario-Abejon, Collin, McKay, & Segal, 1998; Yamada et al., 2002).
Finally, we find it noteworthy that in several brain regions evaluated in our study, BDNF protein levels were lower, and NGF protein levels were higher, in 24- versus 4-month-old male and female rats. This is especially interesting considering that many studies examining the brains of AD patients show a similar profile, with BDNF decreased and NGF increased in the hippocampus and specific neocortical regions (for review see Murer, Yan, & Raisman-Vozari, 2001, or Siegal & Chauhan, 2000).
Acknowledgments
The authors wish to thank the Core Endocrinology Laboratory at Pennsylvania State University for performing the hormone assays. This research was funded by grants awarded to HAB-N (National Institute on Aging [AG026137 and AG026137] and to ACG (AG10755).
Contributor Information
Heather A. Bimonte-Nelson, Department of Psychology, Arizona State University; and Arizona Alzheimer’s Consortium, Tempe, Arizona, USA
Ann-Charlotte E. Granholm, Department of Neuroscience and the Center on Aging, Medical University of South Carolina, Charleston, South Carolina, USA
Matthew E. Nelson, Department of Neuroscience and the Center on Aging, Medical University of South Carolina, Charleston, South Carolina, USA
Alfred B. Moore, Department of Neuroscience and the Center on Aging, Medical University of South Carolina, Charleston, South Carolina, USA
REFERENCES
- Albeck D, Mesches M, Juthberg S, Browning M, Bickford P, Rose G, Granholm A-C. Exogenous NGF restores endogenous NGF distribution in the brain of the cognitively impaired aged rat. Brain Research. 2003;967(1–2):306–310. doi: 10.1016/s0006-8993(03)02272-8. [DOI] [PubMed] [Google Scholar]
- Alberch J, Perez-Navarro E, Arenas E, Marsal J. Involvement of nerve growth factor and its receptor in the regulation of the cholinergic function in aged rats. Journal of Neurochemistry. 1991;57:1483–1487. doi: 10.1111/j.1471-4159.1991.tb06342.x. [DOI] [PubMed] [Google Scholar]
- Backman C, Rose GM, Hoffer BJ, Henry MA, Bartus RT, Friden P, Granholm AC. Systemic administration of a nerve growth factor conjugate reverses age-related cognitive dysfunction and prevents cholinergic neuron atrophy. Journal of Neuroscience. 1996;16:5437–5442. doi: 10.1523/JNEUROSCI.16-17-05437.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA, Yackel JW, Puri PS. Neurons in the rat dentate gyrus granular layer substantially increase during juvenile and adult life. Science. 1982;216:890–892. doi: 10.1126/science.7079742. [DOI] [PubMed] [Google Scholar]
- Bimonte H, Nelson M, Granholm AC. Age-related deficits as working memory load increases: relationships with growth factors. Neurobiology of Aging. 2002;24:37–48. doi: 10.1016/s0197-4580(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Crutcher KA, Weingartner J. Hippocampal NGF levels are not reduced in the aged Fischer 344 rat. Neurobiology of Aging. 1991;12:449–454. doi: 10.1016/0197-4580(91)90072-r. [DOI] [PubMed] [Google Scholar]
- Granholm AC. Oestrogen and nerve growth factor—neuroprotection and repair in Alzheimer’s disease. Expert Opinions in Investigative Drugs. 2000;9:1–10. doi: 10.1517/13543784.9.4.685. [DOI] [PubMed] [Google Scholar]
- Hellweg R, Fischer W, Hock C, Gage FH, Bjorklund A, Thoenen H. Nerve growth factor levels and choline acetyltransferase activity in the brain of aged rats with spatial memory impairments. Brain Research. 1990;537:123–130. doi: 10.1016/0006-8993(90)90348-f. [DOI] [PubMed] [Google Scholar]
- Henriksson BG, Soderstrom S, Gower AJ, Ebendal T, Winblad B, Mohammed AH. Hippocampal nerve growth factor levels are related to spatial learning ability in aged rats. Behavior and Brain Research. 1992;48:15–20. doi: 10.1016/s0166-4328(05)80134-2. [DOI] [PubMed] [Google Scholar]
- Kaisho Y, Ohta H, Miyamato M, Igarashi K. Nerve growth factor promoter driven neurotrophin-3 overexpression in the mouse and the neuro-protective effects of transgene on age-related behavioral deficits. Neuroscience Letters. 1999;277:181–184. doi: 10.1016/s0304-3940(99)00874-5. [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R, Takeuchi IK, Semba R, Kato K. Distribution of brain derived neurotrophic factor in rats and its changes with development in the brain. Journal of Neurochemistry. 1997;69(1):34–42. doi: 10.1046/j.1471-4159.1997.69010034.x. [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R, Semba R, Takeuchi IK, Kato K. Age-related changes in levels of brain-derived neurotrophic factor in selected brain regions of rats, normal mice and senescence-accelerated mice: A comparison to those of nerve growth factor and neurotrophin-3. Neuroscience Research. 1998;31:227–234. doi: 10.1016/s0168-0102(98)00040-6. [DOI] [PubMed] [Google Scholar]
- Larkfors L, Ebendal T, Whittemore SR, Persson H, Hoffer B, Olson L. Developmental appearance of nerve growth factor in the rat brain: significant deficits in the aged forebrain. Progress in Brain Research. 1988;78:27–31. doi: 10.1016/s0079-6123(08)60262-9. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Luine VN, Renner KJ, McEwen BS. Sex-dependent differences in estrogen regulation of choline acetyltransferase are altered by neonatal treatments. Endocrinology. 1986;119:874–878. doi: 10.1210/endo-119-2-874. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial-arm maze test in rats. Journal of Neuroscience. 2000;20:7116–7121. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer’s disease and Parkinson’s disease. Progress in Neurobiology. 2001;63:71–124. doi: 10.1016/s0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]
- Narisawa-Saito M, Nawa H. Differential regulation of hippocampal neurotrophins during aging in rats. Journal of Neurochemistry. 1996;67:1124–1131. doi: 10.1046/j.1471-4159.1996.67031124.x. [DOI] [PubMed] [Google Scholar]
- Nishizuka M, Katoh-Semba R, Eto K, Arai Y, Iizuka R, Kato K. Age- and sex- related differences in the nerve growth factor distribution in the rat brain. Brain Research Bulletin. 1991;27:685–688. doi: 10.1016/0361-9230(91)90045-l. [DOI] [PubMed] [Google Scholar]
- Scott SA, Liang S, Weingartner JA, Crutcher KA. Increased NGF-like activity in young but not aged rat hippocampus after septal lesions. Neurobiology of Aging. 1994;15:337–346. doi: 10.1016/0197-4580(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Siegel GJ, Chauhan N. Neurotrophic factors in Alzheimer’s and Parkinson’s disease brains. Brain Research Reviews. 2000;33:199–227. doi: 10.1016/s0165-0173(00)00030-8. [DOI] [PubMed] [Google Scholar]
- Sugaya K, Greene R, Personett D, Robbins M, Kent C, Bryan D, Skiba E, Gallagher M, McKinney M. Septo-hippocampal cholinergic and neurotrophin markers in age-induced cognitive decline. Neurobiology of Aging. 1998;19:351–361. doi: 10.1016/s0197-4580(98)00072-4. [DOI] [PubMed] [Google Scholar]
- Taglialatela G, Robinson R, Gegg M, Perez-Polo JR. Nerve growth factor, central nervous system apoptosis, and adrenocortical activity in aged Fischer-344/brown Norway F1 hybrid rats. Brain Research Bulletin. 1997;43:229–233. doi: 10.1016/s0361-9230(97)00001-4. [DOI] [PubMed] [Google Scholar]
- Vicario-Abejon C, Collin C, McKay RD, Segal M. Neurotrophins induce formation of functional excitatory and inhibitory synapses between cultured hippocampal neurons. Journal of Neuroscience. 1998;18:7256–7271. doi: 10.1523/JNEUROSCI.18-18-07256.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf N. Cholinergic systems in mammalian brain and spinal cord. Progress in Neurobiology. 1991;37:475–524. doi: 10.1016/0301-0082(91)90006-m. [DOI] [PubMed] [Google Scholar]
- Yamada MK, Nakanishi K, Ohba S, Nakamura T, Ikegaya Y, Nishiyama N, Matsuki N. Brain-derived neurotrophic factor promotes the maturation of GABAergic mechanisms in cultured hippocampal neurons. Journal of Neuroscience. 2002;22:7580–7585. doi: 10.1523/JNEUROSCI.22-17-07580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurek DM, Fletcher-Turner A. Differential expression of GDNF, BDNF, and NT-3 in the aging nigrostriatal system following a neurotoxic lesion. Brain Res.earch. 2001;891:228–235. doi: 10.1016/s0006-8993(00)03217-0. [DOI] [PubMed] [Google Scholar]