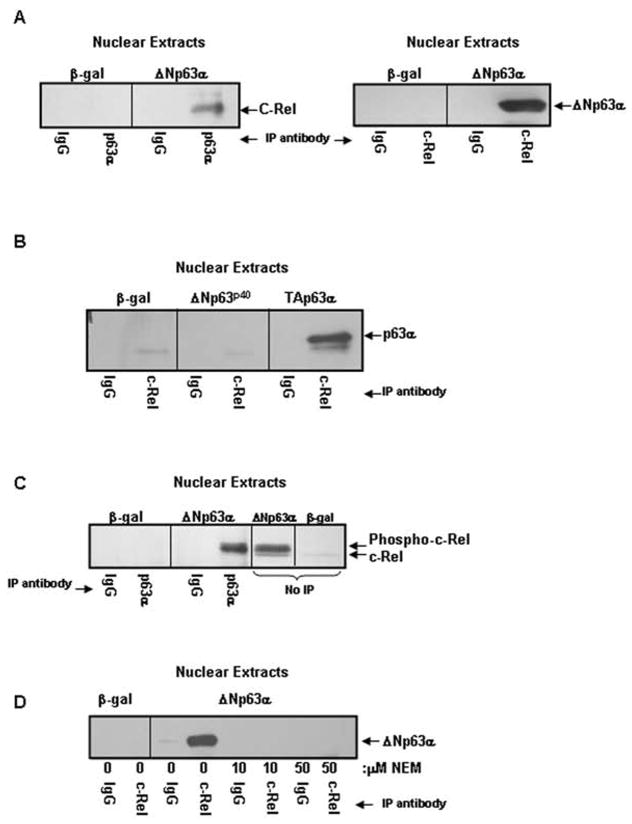
Figure 4. ΔNp63α and c-Rel physically interact in a phosphorylation, p63 α-domain dependent manner.
A) ΔNp63α and c-Rel physically interact in the nuclei of ΔNp63α overexpressing keratinocytes. Co-immunoprecipitation analysis of nuclear extracts from keratinocytes overexpressing ΔNp63α or β-gal. Nuclear extracts were immunoprecipitated with antibody to p63 (left panel) or c-Rel (right panel) and probed for c-Rel or p63, as noted. B) The α-domain but not the ΔN-domain of p63 is required for the interaction between p63 and c-Rel. Nuclear extracts from keratinocytes overexpressing ΔNp63p40 (a truncated form of ΔNp63 lacking the α-COOH-terminus), TAp63α, or β-gal were immunoprecipitated with antibody to c-Rel and probed for p63. C) ΔNp63α associates with phosphorylated c-Rel. Co-immunoprecipitation analysis of nuclear extracts from keratinocytes overexpressing ΔNp63α or β-gal resolved alongside ΔNp63α or β-gal nuclear extracts (no-IP). Co-migration and western blot reveals that the upper, phosphorylated species of c-Rel interacts with ΔNp63α. D) Protein phosphorylation is required for association between ΔNp63α and c-Rel. Co-immunoprecipitation analysis of nuclear extracts from keratinocytes overexpressing ΔNp63α or β-gal. Culture with NEM for one day following adenoviral infection to block in vivo phosphorylation eliminates the interaction between ΔNp63α and c-Rel.