Figure 4.
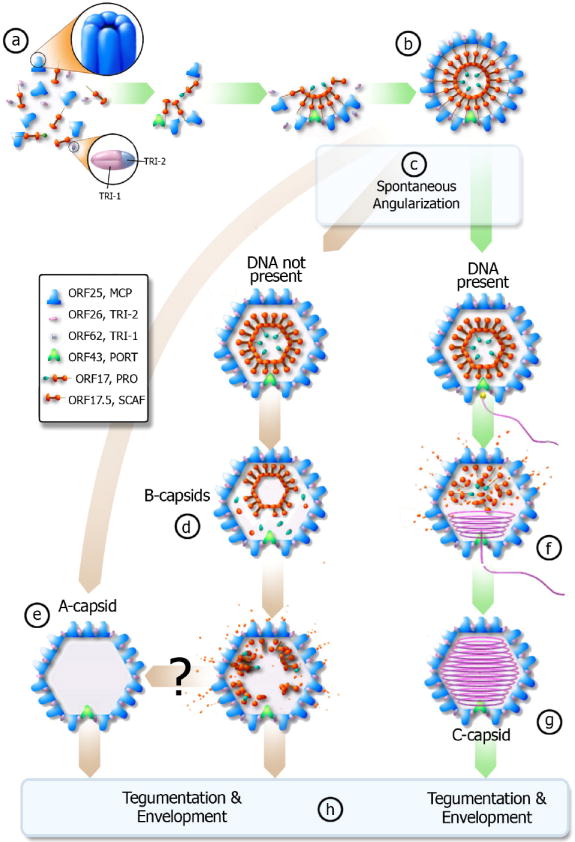
Proposed pathway of KSHV capsid assembly pathway – green arrows denote productive pathway (can create infectious virion), brown arrows denote unproductive pathway. (a) The scaffolding protein (orange) associates with MCP (blue). The scaffolding proteins then interact with each other and pull the MCP together through the “rope” mechanism (see text). Triplexes (pink and blue) bind the MCP together to form a spherical procapsid (b). If DNA is present and available when the procapsid spontaneously angularizes (c), then the DNA will enter the capsid via the portal complex (f) and the scaffold protein will evacuate the capsid to form a C-capsid (g). If the cleavage occurs prematurely and DNA is not present, the cleavage product can likely exit from the porous procapsid shell to result in A-capsid (e). If cleavage occurs slower than angularization, the scaffolding core will likely collapse and some scaffold protein will still evacuate the capsid while forming B-type capsid intermediates (d). It remains unclear whether B-capsid can eventually become an empty A-capsid (e). C-capsids will then obtain a tegument layer and an envelope to become an infectious virion while A and B-capsids lead to non-infectious enveloped particles (h).
