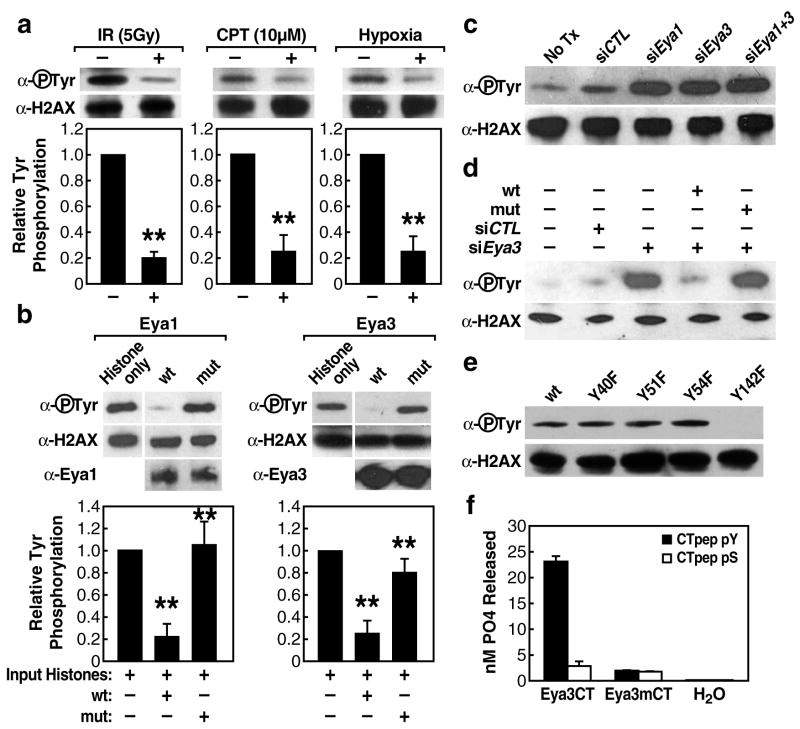
Figure 4.
Tyrosine phosphorylated H2AX is a substrate for Eya phosphatase. (a) IP-western of tyrosine phosphorylated H2AX in response to DNA-damage signals. Bars represent quantified western blot signals normalized to untreated cells. (b) In-vitro phosphatase assay using immunopurified wild type Eya1/3 or enzymatically-inactive mutant proteins (Eya1 D323A, Eya3D246A) and bovine histone. Bars represent quantified western blot signals normalized to input. Mean values +/− SEM from triplicate western blot experiments are shown. “**”p-value <.001. (c) siRNA knockdown of endogenous Eya1/3 in 293T cells (48h) and subsequent IP-western for tyrosine phosphorylated H2AX. (d) Rescue of Eya function by co-transfection of human siRNA and murine wild type or enzymatically inactive mutant Eya3 constructs in 293T human embryonic kidney cells reveals loss of H2AX phosphotyrosine mark dependent on Eya phosphatase activity. (e) Individual substitution mutations of four H2AX tyrosine residues followed by IP-western to detect phosphotyrosine. (f) In-vitro phosphatase assay using bacterially expressed Eya3 EYA domain, wild-type or D246A, with purified peptides of the H2AX tail (AA 128–142) phosphorylated at S139 (CTpep pS) or Y142 (CTpep pY) demonstrates that Eya phosphatase activity is specific for phosphotyrosine. The Km value for Eya dephosphorylation of CTpep pY was 0.38mM with a corresponding Kcat/Km value of 0.96M−1min−1. Bar graphs represent mean +/− SEM of nM PO4 released from triplicate phosphatase reactions.