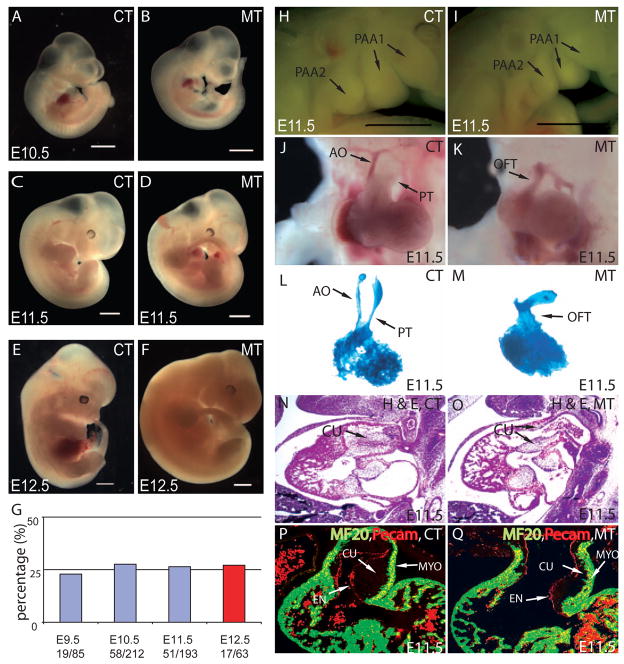
Figure 1. Smad4 deficiency in NCCs results in abnormal OFT septation and severe OFT cushion hypoplasia.
(A–D) From E9.5 to E11.5, except for OFT defects and PA hypoplasia at E11.5, no other morphological difference was overtly apparent between Wnt1Cre;Smad4loxP/loxP mice (MT) and their littermate controls (CT) that do not have homozygous deletion of Smad4 in NCCs. (E,F) Mutant mice died at E12.5. (G) Mutant embryos were recovered at Mendelian ratio (25% from compound heterozygotes intercross) at different developmental stages up to E12.5. All mutants died at E12.5 as evidenced by the absence of heart beat (Red). Scar bar: 1mm. (H,I) PA hypoplasia was observed in mutants at E11.5. Scar bar: 1mm. (J,K) At E11.5, the OFT has developed into pulmonary trunk (PT) and ascending aorta (AO) in the control, while the OFT of the mutant was still not divided. (L,M) Casting experiments revealed two separated outlet channels in the OFT from the control but only one common outlet in the mutant. (N,O) Haematoxylin and Eosin (H&E) staining of sagittal sections at E11.5 showed severe OFT cushion (CU) hypoplasia and persistent truncus arteriosus (PTA) in mutant compared to the control. (P,Q) MF20 staining (green) and Pecam staining (red) of frontal section at E11.5 revealed a thinner layer of cushion in mutant OFT compared to the control.