Summary
Precursors of the hormone gastrin, progastrin and glycine-extended gastrin (G-gly), have been detected in colorectal polyps and tumours, and in the blood of patients with colorectal cancer (CRC), while their expression is lower in healthy subjects. The surface glycoproteins CD133 and CD44 have been identified as possible markers for CRC stem cells. Our aims were to investigate whether progastrin and G-gly are expressed by CD133-positive cells in human CRC tissues and in the human CRC cell line DLD-1, and to determine whether this expression is biologically relevant. The great majority of the cells expressing CD133 also expressed gastrin precursors in both DLD-1 cells, which retain a stem cell-like subpopulation, and human CRC specimens. The CD133high/CD44high/progastrinhigh cells gave rise to larger tumours in SCID mice compared to CD133low/CD44low/progastrinlow cells. The CD133high/CD44high/progastrinhigh cells displayed enhanced activation of the signalling molecules JAK2, STAT3, ERK1/2 and Akt, known to regulate the induction of proliferation and/or survival by gastrin precursors. Moreover, downregulation of the gastrin gene in DLD-1 cells reduced the expression of cancer stem cell markers and abolished tumour development in SCID mice. We conclude that gastrin precursors may provide a target for therapies directed against the cells responsible for tumour development and recurrence.
Keywords: CD133, cancer stem cell, tumour-initiating cell, colon, cancer, gastrin
1. Introduction
Surgery is a frequent form of treatment for colorectal cancer (CRC) with a success rate of approximately 40% over a 5 year period [1]. However, recurrence following surgery is a major problem and is often the ultimate cause of death. Despite the reputation of CRC as a curable disease, and a thorough characterization of the mutations involved in the adenoma-carcinoma sequence [2]; [3]; [4] the observation that CRC remains the second most common cause of cancer-related death worldwide suggests that currently available treatments are unable to eliminate all the cancer cells [5]; [6].
The growth and behaviour of tumours is determined by a small subpopulation of cancer stem cells, which are able to proliferate extensively and differentiate into the heterogeneous groups of cells that form the tumour [7]. These cancer stem cells are resistant to current therapies targeting the tumour, and are thus responsible for the recurrence of the pathology [7]; [8]. Indeed the identification of these cancer stem cells, which are also called tumour-initiating cells, is a priority in order to develop an effective treatment that will totally eradicate the cells that are responsible for tumour development and recurrence.
A subpopulation of cells, positive for the cell-surface glycoprotein CD133 and representing only a small fraction of the entire primary colorectal carcinoma mass, has been independently identified by two groups as cancer-initiating cells [9]; [10]. The CD133-positive cells are able to maintain themselves in culture in an undifferentiated state, to initiate tumour growth after xenotransplantation in immunodeficient mice, and to differentiate into tumours that are phenotypically identical to the original CRC tissue. In contrast, the CD133-negative cells, which form most of the primary tumour and thus might correspond to other cellular differentiation states, were not able to give rise to tumours. The glycoprotein CD133, which localises to membrane protrusions, is thought to participate in the regulation of membrane topology and is probably not directly related to carcinogenesis per se. The utility of CD133 as a stem cell marker in CRC has recently been challenged [11] (See [12] for a balanced review). Moreover, CD133 is not specific for CRC as it is also found in haematopoietic stem cells, in neural progenitor cells and in cancer stem cells from other tissues including prostate, pancreas and liver [9]; [10]; [13]; [14]; [15]; [16]. More recently other markers, such as CD24, CD44, CD166 and Lgr5, have been also identified for CRC stem cells [17].
The hormone gastrin (Gamide) plays important roles in controlling the digestion of food, and in stimulating regeneration of the mucosal lining of the gastrointestinal tract [18]. Moreover, increased concentrations of the gastrin precursors, progastrin and glycine-extended gastrin (G-Gly), have been observed in the circulation of patients with CRC [19]; [20]; [21]. These peptides are also found in 80 to 90% of colorectal polyps, which represent an early stage in the colorectal adenocarcinoma sequence [22] but are absent from healthy tissue. The precursors represent the majority of the gastrin peptides produced by colorectal tumours while only low concentrations of Gamide are present. Interestingly, the observation that resection of the colorectal tumour induces a decrease in the concentration of the precursors in the blood suggests that the tumour is the source of the peptides [23].
Our aim here was to investigate whether progastrin or G-gly is expressed in CD133-positive CRC cells and, if so, the biological relevance of gastrin expression. We first investigated the co-expression of both gastrin precursors, progastrin and G-gly, with the CRC stem cell marker CD133 in human primary colorectal tumour tissues at different stages of differentiation. We then characterized the expression of gastrins, CD44 and CD133 in the human colorectal tumour cell line DLD-1, which retains a stem cell-like subpopulation. Finally, we studied the biological relevance of the expression of gastrin precursors in the CD133-positive CRC stem cell-like population by down regulation of the gastrin gene.
2. Materials and methods
2.1 Antibodies
Anti-progastrin and anti-G-gly antibodies have been characterized and described previously [21]. Briefly, the anti-progastrin antibody (antiserum 1137) recognises the C-terminal sequence (92–101) of human progastrin. The anti-G-gly antibody (antiserum 7270) recognises the C-terminal sequence of G-gly, but does not cross-react with amidated gastrin or cholecystokinin-gly. Anti-JAK2, anti-phosphoJAK2 (Upstate Biotechnology, Charlottesville, VA, USA); anti-phosphoTyr705STAT3, anti-p42/44MAPK, anti-phospho-p42/44MAPK, anti-Akt, anti-phosphoSer473Akt, anti-GAPDH (Cell Signalling Technology, Genesearch, Arundel, Australia); anti-nanog, anti-STAT3, anti-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA); mouse anti-human CD133/1 and mouse anti-human CD133/1-PE (Miltenyi Biotec Australia Pty. Ltd., North Ryde, NSW); anti-cytokeratin 20 (DAKO, Kingsgrove, Australia); anti-CD44, anti-Lgr5 (Novus Biologicals, Littleton, CO, USA); AlexaFluor488 anti-rabbit and AlexaFluor546 anti-mouse IgG (Molecular Probes, Eugene, OR, USA), mouse IgG1s-PE (BD Biosciences, Scoresby, Australia) were from the indicated suppliers.
2.2 Immunofluorescent staining of tissue-arrays
Tissue-arrays were from BioChain Institute (Hayward, CA, USA). Antigens on dewaxed sections were retrieved by boiling slides in 10 mM citrate buffer (pH 6) using a microwave oven. After serum blocking and application of primary antibodies, bound antibodies were detected with fluorochrome-coupled secondary antibody. Control slides, where the primary antibody was replaced by diluted non-immune rabbit IgG, were checked for non-specific reactivity before assessment of the staining. Slides were mounted in fluorescent mounting medium (DAKO, Kingsgrove, Australia) and analysed on a Leitz DMRBE microscope with a Leica DC camera and Leica DC viewer software. Images were further assembled using Adobe Photoshop software. For comparisons, identical concentrations of antibody were used for all samples and the pictures were taken at identical exposure times, gamma correction and gain values.
2.3 Cell culture and growth conditions
The human colorectal tumour cell line DLD-1 was grown in RPMI 1640 medium supplemented with 10% foetal bovine serum at 37°C in a 95% air, 5% CO2 atmosphere. Since the holoclones were very adherent, in order to recover the entire population of the cell line, cells were dissociated by trypsin treatment and scraping before re-plating. DLD-1 VO and AS cells have been described previously [24].
2.4 Immunofluorescent staining of cells
DLD-1 cells were grown in 12-well plates containing coverslips and examined by phase contrast microscopy as colonies developed. Cells were fixed in 2% paraformaldehyde, permeabilized with ice-cold methanol, and blocked with 1% FBS in PBS (containing 1 mM Ca2+, 1 mM Mg2+). The incubations with primary and secondary antibodies were performed according to standard immunofluorescent methods. Slides were mounted in fluorescent mounting medium (DAKO). Immunofluorescent intensity was analyzed in at least three independent experiments using the image analyzer ImageQuant with Total Lab software (GE Healthcare, Rydalmere, Australia) as previously described [25]. Briefly, the size of region of interest (ROI) for the quantification of the intensity was established on a fully stained zone of a cell from an holoclone. At least 5 measurements were performed on fully stained ROI of the same size for cells from both holoclones and paraclones. The data represent an averaged intensity of the 5 ROI quantified for each type of colony.
2.5 Isolation of the paraclone and holoclone cells
As described previously [26], the DLD-1 cells were plated at very low densities in 200-mm Petri dishes for identification of colonies classified as holoclones (i.e., the putative stem cell colonies predicted to contain cells able both to self-renew and to give rise to amplifying cells) and paraclones. These colonies were isolated with cloning rings, their cells dissociated in trypsin/EDTA, washed and resuspended in PBS, then counted and diluted appropriately for the xenograft experiment.
2.6 Flow cytometry
Cells were harvested using PBS containing EDTA and 2% glucose. The cells were incubated in PBS containing 2% FBS with PE-conjugated anti-human CD133/1 antibody (Miltenyi Biotec Australia), fixed in 2% paraformaldehyde-PBS, and then permeabilized with a 0.125% saponin-PBS buffer, incubated in 2% FBS in PBS with the anti-progastrin or anti-G-gly antibody and then with the AlexaFluor488 anti-rabbit antibody. Mouse IgG1s-PE was used as isotype control. Samples were analysed using a FACSDiva flow cytometer and FACSDiva software (BD Biosciences).
2.7 Western blot analysis
Cells were washed once with PBS and then with buffer A (50 mM HEPES, pH 7.5, containing 150 mM NaCl, 10 mM EDTA, 10 mM Na4P2O7, 100 mM NaF, 2 mM Na3VO4). Cells were homogenized in lysis buffer (buffer A containing 1% Nonidet P 40, 0.5 mM PMSF, 20 μM leupeptin, 10 μg/ml aprotinin) for 15 min at 4°C. Proteins were separated by SDS–PAGE and transferred to nitrocellulose membranes. Membranes were blotted with the indicated antibodies. Proteins were visualized using an ECL kit (GE Healthcare).
2.8 Xenografts
Animals were studied under protocols approved by the Austin Health Animal Ethics Committee. SCID mice were inoculated subcutaneously on either hind flank with 50μL PBS containing 5×106 DLD-1 cells (Holoclone or paraclone cells, VO or AS cells) as indicated in Figures 2 and 6. Mice were monitored for tumour development for 21 days, and then humanely killed. Xenografts were excised and tumour volume was measured with digital calipers, using the formula: volume = (length × width2)/2.
Fig. 2. DLD-1 cells retain a stem cell subpopulation.
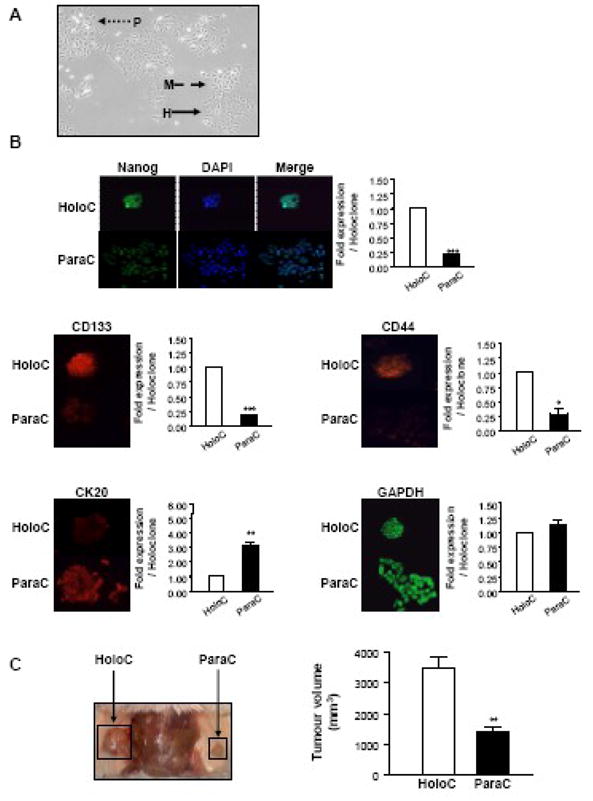
A: Morphology. Photograph of the DLD-1 cell line in culture (Original magnification 40X). A holoclone (H), meroclone (M) and paraclone (P) are indicated by the plain, dashed and dotted arrows, respectively.
B: Immunofluorescent staining. DLD-1 cells were grown in 6-well plates containing coverslips. Cells were fixed, permeabilized and stained with DAPI and antibodies against the stem cell markers Nanog, CD133 and CD44, the differentiation marker cytokeratin 20, or the housekeeping protein GAPDH, using standard immunofluorescent techniques. Slides were analysed as described in the legend to Figure 1. Immunofluorescent intensity was analyzed using the image analyzer Biocom as previously described [25]. Representative photographs from one of three independent experiments are shown (original magnification 50X). Data represent the mean ± SEM (n = 3) and are expressed as fold stimulation of expression compared to holoclone. *** P<0.0001; ** 0.001<P<0.01; *0.01<P<0.05; ns P>0.05 (Students’ t-test). HoloC, holoclone; ParaC, paraclone.
C: Tumorigenicity. SCID mice were inoculated subcutaneously with holoclone or paraclone cells on opposite hind flanks, and monitored for tumour development. After 21 days xenografts were excised and tumour volume was measured with digital callipers. Data are presented as means ± SEM. (**: P = 0.003; n = 4).
Fig. 6. Down regulation of the gastrin gene in DLD-1 cells modulates DLD-1 differentiation and induces a dramatic decrease in CD133 expression.
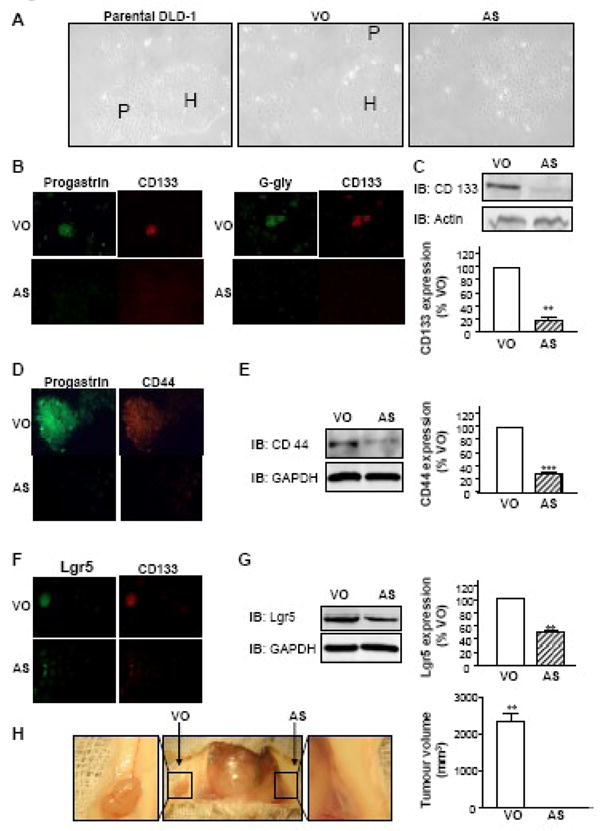
A: Morphology. Photographs of the parental DLD-1 cell line, and the DLD-1 cells transfected either with vector only (VO) or with an antisense (AS) gastrin plasmid in culture (Original magnification 40X). H: holoclones; P: paraclones.
B, D, F: Immunofluorescent staining. DLD-1 cells transfected with vector only (VO) or with an antisense (AS) gastrin plasmid were grown in 6-well plates containing coverslips. Cells were fixed and stained with the indicated antibodies using standard immunofluorescent techniques (original magnification 20X for B and F; 50X for D). Slides were analysed as described in the legend to Figure 1. Representative photographs from three independent experiments are shown.
C, E, G: Western-blot analysis. Lysates of DLD-1 VO cells and AS cells, containing identical protein concentrations, were separated by SDS-PAGE and analysed by western-blot with the antibodies against CD133, CD44 or Lgr5. The membrane was also probed with antibodies against actin or GAPDH to correct for unequal loading of proteins. Data are presented as means ± SEM. IB: Immunoblot.
H: Tumorigenicity. SCID mice were inoculated subcutaneously on either hind flank with VO or AS cells as indicated, and monitored for tumour development. After 21 days xenografts were excised and tumour volume was measured with digital callipers. Data is presented as means ± SEM. (**: P = 0.01; n = 4).
2.9 Statistical analysis
Means (± SEM) were calculated and Students’ t-tests were performed using GraphPad Prism (GraphPad, San Diego, CA, USA). The statistical significance is indicated by the following symbols: ***, P < 0.001; **, 0.001 < P < 0.01; *, 0.01 < P < 0.05; ns: P > 0.05.
3. Results
3.1 Co-expression of gastrin precursors and the CRC stem cell marker CD133 in human primary colorectal tumours
CD133 expression is not restricted to stem cells. However, different epitopes of CD133 with differing glycosylation states have been identified, and the AC133/1 epitope, which was used for the characterization of the CRC stem cells from primary tumours in two previous studies [9]; [10], was shown to be specifically highly expressed in stem cells or progenitor cells. Furthermore expression of the AC133/1 antigen varies as a function of the cellular differentiation state and is restricted to non-differentiated cells [27].
The co-expression of progastrin or G-gly with CD133 was first investigated by co-immunofluorescent staining of a CRC tissue array which contained sixty specimens from primary tumours with different differentiation stages (Table 1). Progastrin, G-gly and CD133 staining was observed in 48, 34 and 39 tumours, respectively. In most of the CRC specimens, progastrin and G-gly were produced by several cells in the mucosa while only a few cells expressed CD133 (Fig. 1). However, most of the cells that expressed CD133 also stained for progastrin or G-gly. Indeed, co-staining for progastrin or G-gly and CD133 was found in 37 (94.9%) or 33 (84.6%) specimens, respectively, out of the 39 tumour specimens staining for CD133. The proportion of co-stained cells was higher in moderately and poorly differentiated tumours than in well differentiated tumours. When staining was observed, the co-expression of gastrin precursors and CD133 in the same cells was even more obvious in moderately and poorly differentiated tissues (Fig. 1). This finding shows that the great majority of the CD133-positive colorectal tumour cells express gastrin precursors throughout the different stages of the development of human colorectal cancers.
Table 1.
Co-expression of gastrin precursors and the colon cancer stem cell marker CD133 in human colorectal tumours.
| Number of positive Tumours | ||||||
|---|---|---|---|---|---|---|
| Differentiation | Staining | ProG | G-gly | CD133 | Co-Staining ProG & CD133 | Co-Staining G-gly & CD133 |
| Number Trs | ||||||
| Well | 24 | 20 83.2% |
15 62.4% |
17 70.7% |
16 66.6% |
14 58.2% |
| Moderately | 22 | 16 72.6% |
12 54.5% |
14 63.6% |
13 59% |
12 54.5% |
| Poorly | 10 | 8 80% |
3 30% |
4 40% |
4 40% |
3 30% |
| ND | 4 | 4 100% |
4 100% |
4 100% |
4 100% |
4 100% |
| TOTAL | 60 |
48 80% |
34 56.7% |
39 65% |
37 61.7% |
33 55% |
ProG: Progastrin, Number Trs: Number of Tumours, ND: Not Determined.
Fig. 1. Co-expression of gastrin precursors with the stem cell marker CD133 in human colorectal cancers.
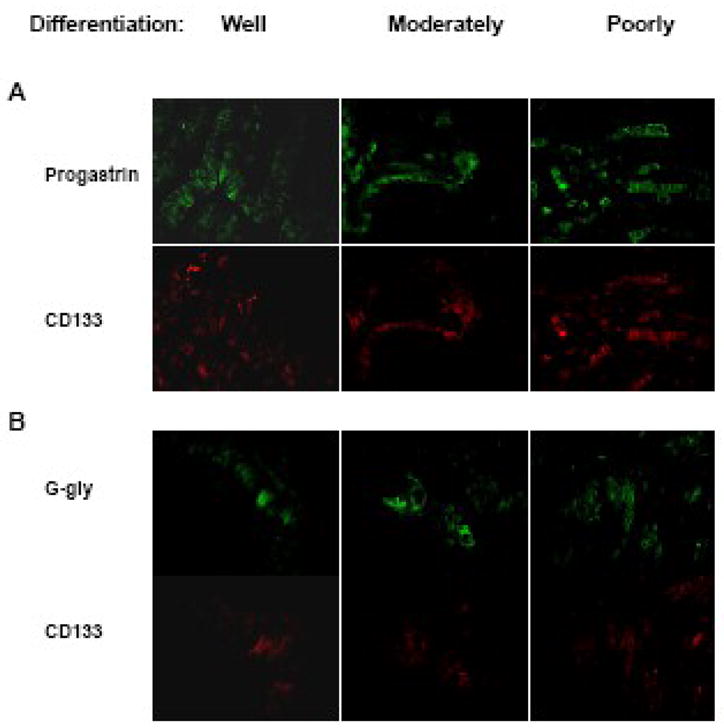
Tissue arrays of human colorectal tumour samples (BioChain Institute, Hayward, CA, USA) were fixed and stained with the indicated antibodies against CD133 and progastrin (A) or G-gly (B) using standard immunofluorescent techniques. Slides were analysed on a Leitz DMRBE microscope with a Leica DC camera and viewer software. For comparisons, identical concentrations of antibody were used for all samples and the pictures were taken at identical exposure times, and gamma correction and gain values. Photographs from representative fields for each differentiation state are shown. (Original magnification 50X.)
3.2 The human CRC cell line DLD-1 retains a stem cell subpopulation
In order to investigate the biological relevance of expression of gastrin precursors by the CD133-positive CRC cells, we used the human CRC cell line DLD-1, which retains a stem cell subpopulation (Fig. 2). As described recently for several cancer cell lines [26]; [28]; [29] the DLD-1 cell line displayed a range of colony morphologies, from holoclones (H-plain arrow) formed by small, densely packed cells, to paraclones (P-dotted arrow) formed by spreading, loosely packed, irregular cells and representing the majority of the cell population (Fig. 2A). Meroclones (M-dashed arrow) corresponded to an intermediate morphology. Moreover, as was also reported in the above mentioned studies [26]; [28]; [29] the holoclones were more adherent than the other clones and were able, after clonal expansion, to give rise to the entire cell population including the three different clonal morphologies (data not shown). Finally, when stained with DAPI in order to visualize the nucleus (Fig. 2B), holoclones had small uniform nuclei while the paraclones displayed variable nuclear size, shape and spacing as previously described. As in the previous studies these different criteria were used to discriminate holoclones, which have been identified as the stem cell subpopulation [26]; [28]; [29], from the paraclones, which were considered to be late-amplifying cells. Holoclones have been reported to display a stronger staining than paraclones for proteins shown to be more highly expressed in normal stem cells [26]; [28]; [29].
In order to further confirm the stem cell character of the cells forming the holoclones, DLD-1 cells were stained for Nanog. Maintenance of a stem cell subpopulation through multiple passages of cells indicates the persistence of the key stem cell property of asymmetrical division [7] Nanog is a transcription factor, localized to the cell nucleus, which plays key roles in the self-renewal and maintenance of pluripotency in embryonic stem cells [30]. Nanog overexpression in murine embryonic stem cells is shown in Fig. 2B, Nanog expression was more than four fold higher in holoclones compared to paraclones (P=0.0002, n=3). Merging of the DAPI and Nanog immunofluorescence confirmed the nuclear localisation of Nanog.
Interestingly, as reported by Ricci-Vitiani and co-workers for the CD133-positive CRC stem cells purified from human tumour tissues [9] the holoclones displayed a high expression of CD133 and low expression of the differentiation marker cytokeratin 20. In contrast the paraclones that stained poorly for CD133 showed good staining for cytokeratin 20, as described previously for the CD133-negative colorectal tumour cells [9]. CD44 has also been reported to be highly expressed in the CRC stem cell population [31]; [32]. In agreement with these observations, holoclones showed a stronger staining than paraclones for this CRC stem cell marker (P=0.003, n=3). No difference between the different clone types was observed for GAPDH expression (P=0.34, n=3) (Fig. 2B).
Finally, the tumorigenicity of cells isolated either from holoclones or paraclones was compared in SCID mice. After isolation of cells from the different clones as previously described [26], cells were injected on either flank of the same animal (Fig. 2C), and tumour growth was followed. After 21 days the tumours obtained from holoclone-derived cells, which stained strongly for CD133 and CD44, were significantly bigger in each mouse than the tumours arising from paraclone-derived cells, which stained only weakly for CD133 and CD44. The observation that cells forming the holoclones have a greater capacity to give rise to tumours than the cells forming the paraclones is consistent with the conclusion that the holoclones represent a stem cell-like population.
3.3 Gastrin precursors are mainly expressed in the stem cell-like population of the human tumour cell line DLD-1
The gastrin precursors, progastrin and G-gly, have been detected in CRC and in the blood of patients with CRC although they are absent from healthy colorectal tissue [22]. Moreover, the observation that tumour resection induced a decreased concentration of gastrin peptides in the blood of CRC patients suggested that the peptides were synthesized by the tumour itself. We previously detected the expression of significant amounts of progastrin and G-gly, but only a negligible amount of Gamide, in DLD-1 cells by radioimmunoassay [24]. Gastrin precursors have also been shown to promote the proliferation of this cell line [33].
DLD-1 cells were therefore stained for gastrin peptides by immunofluorescence. As shown at 10X (Fig. 3A) and 50X (Fig. 3B) magnification, holoclones displayed a significantly stronger expression of both progastrin (10 fold higher P<0.0001, n=3) and G-gly (four fold higher P=0.0004, n=3) (Fig. 3B bottom panel) than paraclones which stained very poorly, if at all. Meroclones showed an intermediate expression of both peptides. In agreement with the previous radioimmunoassay data, DLD-1 cells stained only very weakly for the mature hormone, Gamide, and no difference was observed between the different clone types.
Fig. 3. Gastrin precursors are highly expressed in the stem cell subpopulation.
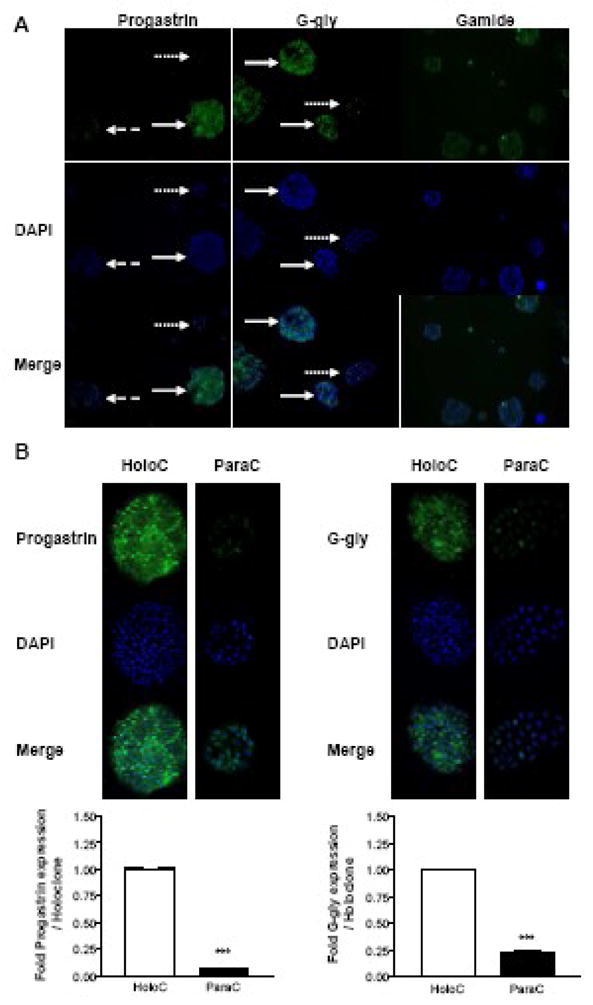
DLD-1 cells were grown in 6-well plates containing coverslips. Cells were fixed, permeabilized and stained with the indicated antibodies and DAPI using standard immunofluorescent techniques. Slides were analysed as described in the legend to Figure 1. Immunofluorescent intensity was analyzed using the image analyzer Biocom as previously described [25]. Representative photographs from one of three independent experiments are shown at an original magnification of 10X (A) or 50X (B). Plain, dashed and dotted arrows represent holoclones, meroclones and paraclones, respectively. Data represent the mean ± SEM (n=3) and are expressed as fold stimulation of expression compared to holoclone. *** P<0.0001; ** 0.001<P<0.01; *0.01<P<0.05; ns P>0.05 (Students’ t-test).
3.4 Specific activation in holoclones of the signalling pathways responsible for growth-promotion by gastrin precursors in vivo
The in vivo activation of JAK2, STAT3, ERK and Akt has been described previously in the proliferating colonic mucosa of transgenic hGAS and MTI/G-gly mice, which overexpress progastrin or G-gly, respectively [25]. Blockade of these pathways resulted in a decreased proliferation/survival rate of isolated colonic epithelial cells in primary culture [25]. The expression and activation levels of these molecules were therefore analysed by immunofluorescent microscopy of DLD-1 cells using antibodies specific either for the total or the activated form of these proteins (Fig. 4). No major difference was observed in the expression levels of total JAK2, ERK and Akt between holoclones and paraclones. However, the expression of the transcription factor STAT3 was significantly higher in holoclones than in paraclones (P=0.007, n=3). Moreover, holoclones consistently displayed a significantly higher staining of the activated forms of JAK2, STAT3, ERK and Akt. It was particularly interesting to note that in the holoclones the active tyrosine-phosphorylated form of the transcription factor STAT3 was detectable, by merging of the DAPI and phospho-STAT3 immunofluorescence, in the nucleus where it regulates gene transcription (Fig. 4).
Fig. 4. Holoclones display greater activation of JAK2, STAT3, ERK and Akt than paraclones.
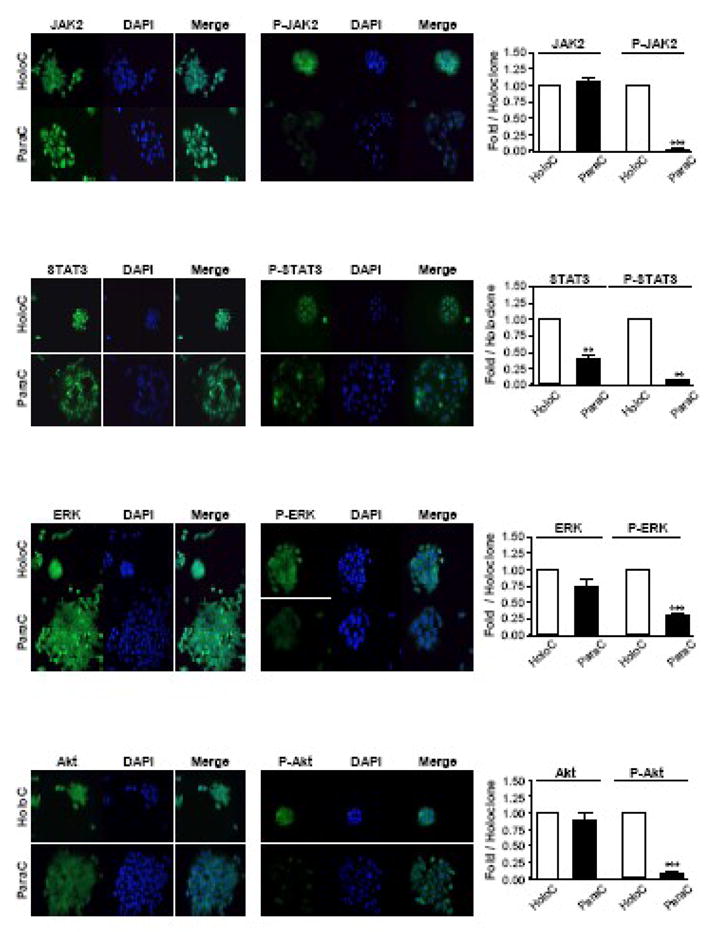
DLD-1 cells were grown in 6-well plates containing coverslips. Cells were fixed, permeabilized and stained with the indicated antibodies and DAPI using standard immunofluorescent techniques. Slides were analysed as described in the legend to Figure 1. Representative micrographs from one of three independent experiments are shown (Original magnification 50X). Data represent the mean ± SEM (n = 3) and are expressed as fold stimulation of expression compared to holoclone. *** P<0.0001; ** 0.001<P<0.01; *0.01<P<0.05; ns P>0.05 (Students’ t-test).
3.5 Correlated expression of gastrin precursors and the CRC stem cell marker CD133 in DLD-1 cells
Since the holoclones highly expressed both CD133 and gastrin precursors, the coexpression of CD133 and progastrin or Ggly in DLD-1 cells was analysed by immunofluorescence with appropriate antibodies (Fig. 5A). The cells were fixed in 2% paraformaldehyde-PBS, stained for CD133 using the antibody directed against the AC133/1 antigen, and then permeabilized and stained for gastrin precursors. Strong co-staining of CD133 and both gastrin precursors was observed in the holoclones which contain a stem cell-like population, while weak or no co-staining was found in the meroclones and paraclones, respectively.
Fig. 5. Co-expression of gastrin precursors with the CRC stem cell marker CD133 in DLD-1 cells.
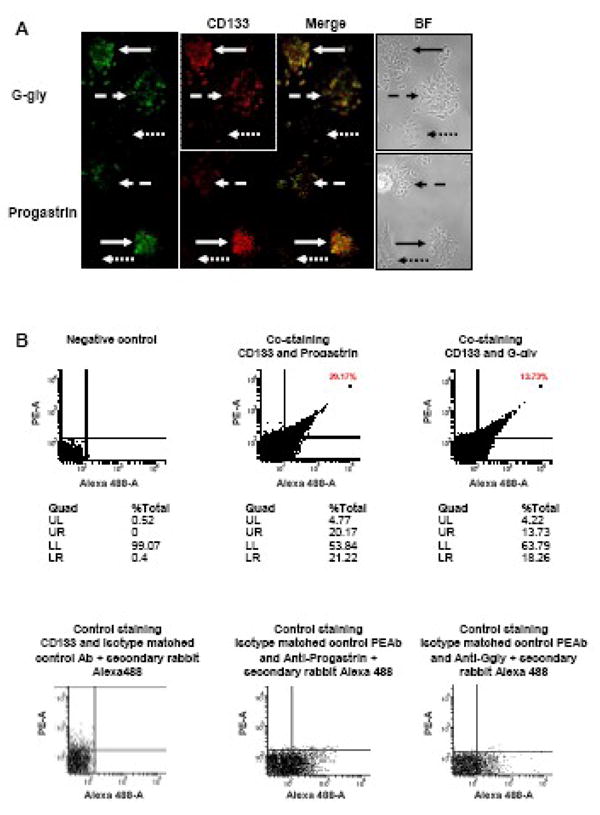
A: DLD-1 cells were grown in 6-well plates containing coverslips. Cells were fixed, stained for CD133, and then permeabilized and stained with the indicated gastrin precursor antibodies using standard immunofluorescent techniques. Slides were analysed as described in the legend to Figure 1. Representative micrographs from three independent experiments are shown. BF: Bright Field photography. Holoclones, meroclones and paraclones are indicated by the plain, dashed and dotted arrows, respectively (Original magnification 50X).
B: FACS analysis of the expression of gastrin precursors and CD133 in DLD-1 cells. The cells were fixed in 2% paraformaldehyde-PBS, stained with the anti-CD133/1-PE antibody, and then permeabilized and stained for gastrin precursors which were detected using rabbit anti-human primary antibodies and an anti-rabbit Alexa488 antibody. Negative controlsamples were used to set gates for further analysis, such that 99% of the total population was present in the lower left quadrant of the dot-plot. One representative experiment from three independent experiments is shown. UL: Upper Left, UR: Upper Right, LL: Lower Left, LR: Lower Right.
In order to further characterize the co-expression of progastrin or G-gly with CD133, a FACS analysis was performed (Fig. 5B). The cells were stained for cell surface CD133 using the antibody directed against the AC133/1 antigen, fixed in 2% paraformaldehyde-PBS, and then permeabilized and stained for cytoplasmic gastrin precursors. Cell staining was analysed cytofluorimetrically (Fig. 5B). Negative control samples were used to set gates for further analysis, such that 99% of the total population was in the lower left quadrant. As shown for a representative experiment in Fig. 5B, approximately 20% of the total DLD-1 cell population expressed CD133. Moreover, most of the cells expressing CD133 also co-stained for progastrin (18.7 ± 0.8 % of the total DLD-1 cell population, n=3) or G-gly (13.1 ± 1.9 % of the total DLD-1 cell population, n=3). No significant difference was found between the percentage of CD133 co-expression with either progastrin or G-gly (P=0.052). This observation confirms that the holoclones, which correspond to the stem cell-like subpopulation in the tumour cell line, strongly express progastrin and G-gly as well as CD133.
3.6 Reduction of gastrin gene expression induces a dramatic decrease in the expression of the colorectal stem cell markers CD133, CD44 and Lgr5, and inhibits xenograft development in SCID mice
Stable transfection of DLD-1 cells with antisense (AS) gastrin constructs induced a change of morphology, as the AS cell population no longer displayed the three types of clones and all the cells appeared to be widely spread and closely attached together (Fig. 6A), and resulted in a significant, although not complete, reduction of gastrin expression (Fig. 6B). As previously reported, the observation that reduction of gastrin peptide expression restored membrane localisation of zonula occludens-1 (ZO-1), occludin, beta-catenin and E-cadherin [24], suggested a modification of the differentiation of the cells. A number of additional markers of stem cell populations have recently been reported. Haraguchi and coworkers have demonstrated that within CRC cell lines, only the CD133-positive CD44-positive population is able to produce tumours [32] while recent studies in mice suggest that the G-protein-coupled receptor Lgr5 might be another marker for both colon stem cells and CRC stem cells [34]. Importantly, the expression of these three markers (CD133, CD44 and Lgr5) was dramatically reduced in AS cells compared to cells transfected with the vector only (VO cells) (Fig. 6B, D, F). The decrease in the expression of these different colon stem cell markers after major depletion of gastrin peptides by transfection of DLD-1 cells with an AS gastrin plasmid was confirmed by western-blot analysis (Fig. 6C, E, G). A significant reduction of CD133, CD44 and Lgr5 was found in AS cells, in which expression was only 18.5±4.0% (P=0.002; n=3), 28.2±2.5% (P=0.0005; n=3), and 50.2±2.1% (P=0.004; n=3), respectively, of their expression in VO control cells.
Two previous studies of the CD133-positive stem cells in primary colorectal tumours [9]; [10] elegantly demonstrated that cells that did not express CD133 lost their ability to develop into tumours when xenografted in immunodeficient mice. A more recent report demonstrated that the CD133-positive CD44-positive population within colorectal tumours was the one able to produce a tumour [32]. The weak expression of CD133, CD44 and Lgr5 in DLD-1 AS cells, as well as the morphological changes observed (Fig. 6A), therefore implied that these cells might have undergone differentiation and lost their stem cell characteristics. To test this hypothesis, the tumorigenicity of AS and VO cells was compared in SCID mice. AS or VO cells were injected on either flank of the same animal (Fig. 6E), and tumour growth was followed. After 21 days while the VO cells had developed into a tumour in each mouse, no tumours had developed from the AS cells. This observation demonstrated that a major reduction of gastrin gene expression in CRC stem cells abolished their capacity to initiate tumours.
4. Discussion
The hypothesis has been proposed during the last decade that cancer tissue, like normal tissue, develops from a stem cell subpopulation that has the abilities to self-renew and to differentiate into multiple cell types. The first evidence of a cancer stem cell subpopulation in tumours came from a study in 1994, when Lapidot and co-workers characterized a subpopulation of CD34+/CD38- cells in acute myeloid leukaemia capable of initiating the human disease in immunodeficient mice [35]. These cells possessed the same differentiative and proliferative capacities, and the same self-renewal potential, as the leukaemic stem cell, and developed into leukaemia that was histologically similar to the human disease. Since 1994, cancer stem cells have also been identified in solid tumours of the breast, brain, prostate, and more recently colon [9]; [10]. Two independent studies have identified the CD133-positive cell subpopulation in CRC as CRC stem cells [9] [10] and a recent study reports that CD133 expression in CRC correlates with a poorer survival [36]. However CD133 is not specific for CRC stem cells and not all CD133-positive cells in colorectal cancers are tumorigenic [9]; [10] More recent studies have identified other markers, such as CD44, CD166, ESA, CD24 and Lgr5, that might be used to identify CRC stem cells [17]. Although these different surface proteins could be useful targets for therapy directed against the CRC stem cells, there is still an urgent need to identify alternative therapeutic targets, that are involved in colorectal tumour development, that are preferably found only in the cancer tissue, and that might specifically regulate the CRC stem cells.
Gastrin precursors are good candidates for such targets. These peptides, which are known to regulate the growth and survival of human colorectal carcinoma cells, are absent from healthy colorectal mucosa but are overexpressed in polyps and tumours [18]. We now report that analysis of sixty primary human colorectal tumours at different differentiation stages revealed the co-expression of progastrin or G-gly with the cancer stem cell marker CD133 in 94.9 % and 84.6 %, respectively, of the specimens. We therefore explored the biological relevance of gastrin precursor expression in the human CRC cell line DLD-1, which has previously been shown by radioimmunoassay to express significant amounts of progastrin and G-gly but only a negligible amount of Gamide [24].
Primary keratinocytes display three typical clonal morphologies represented by holoclones, meroclones, and paraclones [37]. Holoclones contain self-renewing stem cells, while meroclones and paraclones contain more mature and differentiated cells. Interestingly, even after many passages human epithelial cancer cells in clonal cultures also form holoclones, meroclones, and paraclones. Moreover tumour cell holoclones have been shown to harbour stem-like cells or cancer stem cells. Locke et al. have shown that stem cells are also present in head and neck squamous cell carcinomas, the prostate cell line DU145 and the breast cancer cell line MCF7 [26]. More recently, Li and colleagues have demonstrated that holoclones in the human prostate carcinoma cell line PC3 contain self-renewing tumour-initiating cells [29]. Here, we report that the human primary CRC cell line, DLD-1, also displays the same three types of clones. Importantly the holoclones resulted in much larger tumours than paraclones when injected into SCID mice.
The holoclones, when compared to paraclones, strongly express several CRC stem cell markers, including CD133 and CD44. CD133 mRNA expression in the DLD-1 cell line has recently been reported by Ieta and colleagues in a survey of several CRC cell lines [38]. Moreover, as described for CD133-positive cancer stem cells isolated from human CRC [9], the DLD-1 CD133high cells only weakly express the differentiation marker cytokeratin 20. Similarly, the observation of higher staining for stem cell markers by holoclones was made in the breast cancer cell line MCF7 which also displays the three clone morphologies. MCF7 holoclones strongly expressed CD44, the surface marker used to identify the breast cancer stem cells in tumour tissue [39]. Recently, Dalerba et al. reported that CRC cells expressing CD44 also displayed stem cell properties as they were able to engraft in vivo in SCID mice. The resulting tumours maintained a differentiated phenotype and reproduced the entire cellular heterogeneity of their parental lesions [31]. A more recent study showed that within the tumour cells, selection for the CD133-positive CD44-positive population efficiently enriched colorectal stem cells [32]. The DLD-1 cell line expresses CD44 [40] and we demonstrate here that holoclones also display a higher expression of the protein than paraclones (Fig. 2B). We also found in the DLD-1 cell line that the cells highly expressing CD133 and CD44 (the holoclone population) frequently co-express either progastrin or G-gly. Interestingly, the approximate linearity of the relationship between expression of CD133 and progastrin or G-gly (Fig. 5B) suggests that the expression of the two genes may be coordinated. The observation that stable transfection of DLD-1 cells with antisense (AS) gastrin constructs resulted in a dramatic decrease in CD133 expression is consistent with this suggestion (Fig. 6C).
As previously mentioned, JAK2, STAT3, ERK and Akt are activated in vivo in the proliferating colonic mucosa of transgenic mice overexpressing either progastrin or G-gly [25]. Moreover, blockade of these pathways resulted in a decreased proliferation/survival rate of isolated colonic epithelial cells in primary culture [25]. On the other hand, the resistance of cancer stem cells to chemotherapy or radiotherapy is thought to be responsible for tumour recurrence [7]. In this context it is relevant to note that the Akt pathway is well established as an important regulator of cell survival. For instance, the resistance of CD133-positive hepatocarcinoma stem cells to chemotherapy with doxorubicin and 5-fluorouracil has been shown to be preferentially mediated by activation of the Akt pathway [41]. Moreover, the transcription factor STAT3 is now thought to be a predictive marker of resistance to chemotherapy [42]. Inhibition of the STAT3 pathway downregulates the expression of survival proteins and restores cellular sensitivity to signalling by cell-death receptors. Recent studies have reported an abnormally increased STAT3 activation in ovarian cancer cells resistant to paclitaxel treatment [43] and Niu and co-workers have demonstrated that STAT3 plays a protective role in CRC cells in which its inactivation enhances UV-induced cell death [44]. We observed in DLD-1 cells that the stem cell-like subpopulation, which expresses progastrin and G-gly, also displays enhanced activation of the Akt and STAT3 signalling molecules (Fig. 4), which are all known to regulate the proliferation and/or survival induced by gastrin precursors in vitro [45] and in vivo [25]. The evidence is therefore consistent with the hypothesis that gastrin precursors might participate in increasing the survival of the DLD-1 stem cell population by autocrine regulation, and may therefore contribute to the resistance of cancer stem cells to chemotherapy or radiotherapy.
In contrast, downregulation of the expression of gastrin peptides in DLD-1 AS cells correlated with a morphological change and a dramatic decrease in CD133, CD44 and Lgr5 expression. Since down-regulation of CD133 and CD44 upon differentiation has been previously described in the human CRC cell lines Caco-2 [46] and HT29 [32], this observation suggests a reduction in gastrin concentration may have induced differentiation. In this context it is interesting to note that progastrin regulates the beta-catenin/Tcf-4 pathway, a major regulator of the differentiation process in colon [47], and that down-regulation of gastrin increases the numbers of intestinal goblet cells in APC 14 mice [47]. The present observation that the down regulation of the gastrin gene in DLD-1 cells, where gastrin peptides are found predominantly in the CRC stem cell population, prevented the development of tumours in SCID mice (Fig. 6H) further suggests that gastrin gene expression in the CRC stem cell is crucial for its ability to give rise to tumours. Interestingly, since the AS cells can be readily expanded in culture, the absence of tumorigenic activity is not a consequence of loss of the capacity for self-renewal. As the AS cells are still able to proliferate, the dramatic decrease observed in the expression of the cancer stem cell markers, and the loss of their capacity to give rise to tumours, indicate a change in the differentiation status of the AS cells, compared to the holoclone subpopulation within the parental DLD-1 cell line.
The role of CD133 as a CRC stem cell marker has recently been challenged [11]. In contrast to all the previous studies performed on human primary colorectal tumour tissues or cells lines which described the CD133-positive population within the colorectal primary tumours as cancer stem cells [9], [10] the authors argued that CD133 expression in colon is not restricted to stem cells and that both the CD133-positive and -negative cell populations from metastatic tissues are able to give rise to tumours in immunodeficient mice. As discussed by LaBarge and Bissel [12], Shmelkov and coworkers did not actually test the functional tumour-initiating capacity of the CD133-negative populations in the primary tumours they analysed and thus did not conclusively demonstrate that CD133 expression does not segregate with the cancer stem cell population in the primary tumours [11]. Considering the discrepancy between this recent study analysing the cancer stem cell capacity in metastatic tumours and previous reports performed on primary tumours, a difference may exist between the cancer stem cell populations of primary and metastatic tumours. The difference in views regarding the suitability of CD133 as a CRC stem cell marker emphasizes the need to validate additional function molecular markers of CRC stem cells. In regard to the different studies characterising the expression of CD133 in primary tumours, LaBarge and Bissel [12] suggest that a more appropriate marker of the CRC stem cells in the primary tumours would be their level of expression of CD133, with a high level corresponding to a stem cell population. This suggestion is in accordance with our observation within the DLD-1 population where the holoclones strongly express CD133, while the paraclones show only weak expression of the glycoprotein, and the meroclones present an intermediate staining. Interestingly, as shown in Figure 5, we found a correlation of expression between CD133 and gastrin precursors in this cell line. However, downregulation of CD133 in AS cells does not appear to lead to metastatic cells since, as mentioned above, the reduction of gastrin peptide expression strengthen cell-cell adhesion by restoring membrane localisation of zonula occludens-1 (ZO-1), occludin, beta-catenin and E-cadherin [24]. Moreover, tumours were not observed elsewhere in the SCID mice injected with DLD-1-AS cells.
In conclusion, our study demonstrates, in CRC tissues at different pathological stages and in the human CRC cell line DLD-1, that CRC cells which are putatively identified as stem cells on the basis of their high expression of CD133, CD44 and lgr5 also express the gastrin precursors, progastrin and G-gly. Furthermore the ability of DLD-1 cells to grow into tumours is largely dependent on gastrin gene expression. As a consequence, gastrin precursors could represent an excellent therapeutic target in the cells responsible for tumour development and recurrence.
Acknowledgments
We thank Natalie Dodge and Russel Hodgson for their help with the FACS experiments. This work is supported by grant 5 RO1 GM065926-06 from the National Institutes of Health (to GB, AS), grants 400062 (to GB, AS), 208926 (to GB) and 350235 (to AS) from the National Health and Medical Research Council of Australia, grant CT8917 from Medical Research and Technology in Victoria which is managed by ANZ Trustees (to AF), and a grant from the Austin Medical Research Foundation (to AF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fong Y, Cohen AM, Fortner JG, Enker WE, Turnbull AD, Coit DG, Marrero AM, Prasad M, Blumgart LH, Brennan MF. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938–946. doi: 10.1200/JCO.1997.15.3.938. [DOI] [PubMed] [Google Scholar]
- 2.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 3.Fearon ER, Jones PA. Progressing toward a molecular description of colorectal cancer development. Faseb J. 1992;6:2783–2790. doi: 10.1096/fasebj.6.10.1321771. [DOI] [PubMed] [Google Scholar]
- 4.Radtke F, Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science. 2005;307:1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- 5.O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420–1425. doi: 10.1093/jnci/djh275. [DOI] [PubMed] [Google Scholar]
- 6.Nelson H, Petrelli N, Carlin A, Couture J, Fleshman J, Guillem J, Miedema B, Ota D, Sargent D. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst. 2001;93:583–596. doi: 10.1093/jnci/93.8.583. [DOI] [PubMed] [Google Scholar]
- 7.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 8.Al-Hajj M, Becker MW, Wicha M, Weissman I, Clarke MF. Therapeutic implications of cancer stem cells. Curr Opin Genet Dev. 2004;14:43–47. doi: 10.1016/j.gde.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 11.Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T, St Clair R, Baljevic M, White I, Jin DK, Chadburn A, Murphy AJ, Valenzuela DM, Gale NW, Thurston G, Yancopoulos GD, D’Angelica M, Kemeny N, Lyden D, Rafii S. CD133 expression is not restricted to stem cells, and both CD133+ and CD133- metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118:2111–2120. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaBarge MA, Bissell MJ. Is CD133 a marker of metastatic colon cancer stem cells? J Clin Invest. 2008;118:2021–2024. doi: 10.1172/JCI36046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suetsugu A, Nagaki M, Aoki H, Motohashi T, Kunisada T, Moriwaki H. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem Biophys Res Commun. 2006;351:820–824. doi: 10.1016/j.bbrc.2006.10.128. [DOI] [PubMed] [Google Scholar]
- 14.Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, Zheng BJ, Guan XY. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542–2556. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 15.Olempska M, Eisenach PA, Ammerpohl O, Ungefroren H, Fandrich F, Kalthoff H. Detection of tumor stem cell markers in pancreatic carcinoma cell lines. Hepatobiliary Pancreat Dis Int. 2007;6:92–97. [PubMed] [Google Scholar]
- 16.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 17.Vermeulen L, Todaro M, de Sousa Mello F, Sprick MR, Kemper K, Perez Alea M, Richel DJ, Stassi G, Medema JP. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc Natl Acad Sci U S A. 2008;105:13427–13432. doi: 10.1073/pnas.0805706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrand A, Wang TC. Gastrin and cancer: a review. Cancer Lett. 2006;238:15–29. doi: 10.1016/j.canlet.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 19.Kochman ML, DelValle J, Dickinson CJ, Boland CR. Post-translational processing of gastrin in neoplastic human colonic tissues. Biochem Biophys Res Commun. 1992;189:1165–1169. doi: 10.1016/0006-291x(92)92326-s. [DOI] [PubMed] [Google Scholar]
- 20.Nemeth J, Taylor B, Pauwels S, Varro A, Dockray GJ. Identification of progastrin derived peptides in colorectal carcinoma extracts. Gut. 1993;34:90–95. doi: 10.1136/gut.34.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciccotosto GD, McLeish A, Hardy KJ, Shulkes A. Expression, processing, and secretion of gastrin in patients with colorectal carcinoma. Gastroenterology. 1995;109:1142–1153. doi: 10.1016/0016-5085(95)90572-3. [DOI] [PubMed] [Google Scholar]
- 22.Smith AM, Watson SA. Gastrin and gastrin receptor activation: an early event in the adenoma-carcinoma sequence. Gut. 2000;47:820–824. doi: 10.1136/gut.47.6.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konturek PC, Bielanski W, Konturek SJ, Hartwich A, Pierzchalski P, Gonciarz M, Marlicz K, Starzynska T, Zuchowicz M, Darasz Z, Gotze JP, Rehfeld JF, Hahn EG. Progastrin and cyclooxygenase-2 in colorectal cancer. Dig Dis Sci. 2002;47:1984–1991. doi: 10.1023/a:1019652224424. [DOI] [PubMed] [Google Scholar]
- 24.Hollande F, Lee DJ, Choquet A, Roche S, Baldwin GS. Adherens junctions and tight junctions are regulated via different pathways by progastrin in epithelial cells. J Cell Sci. 2003;116:1187–1197. doi: 10.1242/jcs.00321. [DOI] [PubMed] [Google Scholar]
- 25.Ferrand A, Bertrand C, Portolan G, Cui G, Carlson J, Pradayrol L, Fourmy D, Dufresne M, Wang TC, Seva C. Signaling pathways associated with colonic mucosa hyperproliferation in mice overexpressing gastrin precursors. Cancer Res. 2005;65:2770–2777. doi: 10.1158/0008-5472.CAN-04-0978. [DOI] [PubMed] [Google Scholar]
- 26.Locke M, Heywood M, Fawell S, Mackenzie IC. Retention of intrinsic stem cell hierarchies in carcinoma-derived cell lines. Cancer Res. 2005;65:8944–8950. doi: 10.1158/0008-5472.CAN-05-0931. [DOI] [PubMed] [Google Scholar]
- 27.Mizrak D, Brittan M, Alison MR. CD133: molecule of the moment. J Pathol. 2008;214:3–9. doi: 10.1002/path.2283. [DOI] [PubMed] [Google Scholar]
- 28.Wei C, Guomin W, Yujun L, Ruizhe Q. Cancer Stem-like Cells in Human Prostate Carcinoma Cells DU145: The Seeds of the Cell Line? Cancer Biol Ther. 2007;6:763–768. doi: 10.4161/cbt.6.5.3996. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Chen X, Calhoun-Davis T, Claypool K, Tang DG. PC3 human prostate carcinoma cell holoclones contain self-renewing tumor-initiating cells. Cancer Res. 2008;68:1820–1825. doi: 10.1158/0008-5472.CAN-07-5878. [DOI] [PubMed] [Google Scholar]
- 30.Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 31.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, Castelli C, Clarke MF. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haraguchi N, Ohkuma M, Sakashita H, Matsuzaki S, Tanaka F, Mimori K, Kamohara Y, Inoue H, Mori M. CD133+CD44+ population efficiently enriches colon cancer initiating cells. Ann Surg Oncol. 2008;15:2927–2933. doi: 10.1245/s10434-008-0074-0. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed S, Budai B, Heredi-Szabo K, Farkas J, Toth G, Murphy RF, Lovas S. High and low affinity receptors mediate growth effects of gastrin and gastrin-Gly on DLD-1 human colonic carcinoma cells. FEBS Lett. 2004;556:199–203. doi: 10.1016/s0014-5793(03)01408-x. [DOI] [PubMed] [Google Scholar]
- 34.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 35.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 36.Horst D, Kriegl L, Engel J, Kirchner T, Jung A. CD133 expression is an independent prognostic marker for low survival in colorectal cancer. Br J Cancer. 2008;99:1285–1289. doi: 10.1038/sj.bjc.6604664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barrandon Y, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci U S A. 1987;84:2302–2306. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ieta K, Tanaka F, Haraguchi N, Kita Y, Sakashita H, Mimori K, Matsumoto T, Inoue H, Kuwano H, Mori M. Biological and Genetic Characteristics of Tumor-Initiating Cells in Colon Cancer. Ann Surg Oncol. 2007 doi: 10.1245/s10434-007-9605-3. [DOI] [PubMed] [Google Scholar]
- 39.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trejdosiewicz LK, Morton R, Yang Y, Banks RE, Selby PJ, Southgate J. Interleukins 4 and 13 upregulate expression of cd44 in human colonic epithelial cell lines. Cytokine. 1998;10:756–765. doi: 10.1006/cyto.1998.0361. [DOI] [PubMed] [Google Scholar]
- 41.Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133(+) HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2007 doi: 10.1038/sj.onc.1210811. [DOI] [PubMed] [Google Scholar]
- 42.Barre B, Vigneron A, Perkins N, Roninson IB, Gamelin E, Coqueret O. The STAT3 oncogene as a predictive marker of drug resistance. Trends Mol Med. 2007;13:4–11. doi: 10.1016/j.molmed.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Duan Z, Foster R, Bell DA, Mahoney J, Wolak K, Vaidya A, Hampel C, Lee H, Seiden MV. Signal transducers and activators of transcription 3 pathway activation in drug-resistant ovarian cancer. Clin Cancer Res. 2006;12:5055–5063. doi: 10.1158/1078-0432.CCR-06-0861. [DOI] [PubMed] [Google Scholar]
- 44.Niu G, Wright KL, Ma Y, Wright GM, Huang M, Irby R, Briggs J, Karras J, Cress WD, Pardoll D, Jove R, Chen J, Yu H. Role of Stat3 in regulating p53 expression and function. Mol Cell Biol. 2005;25:7432–7440. doi: 10.1128/MCB.25.17.7432-7440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrand A, Kowalski-Chauvel A, Pannequin J, Bertrand C, Fourmy D, Dufresne M, Seva C. Glycine-extended gastrin activates two independent tyrosine-kinases in upstream of p85/p110 phosphatidylinositol 3-kinase in human colonic tumour cells. World J Gastroenterol. 2006;12:1859–1864. doi: 10.3748/wjg.v12.i12.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corbeil D, Roper K, Hellwig A, Tavian M, Miraglia S, Watt SM, Simmons PJ, Peault B, Buck DW, Huttner WB. The human AC133 hematopoietic stem cell antigen is also expressed in epithelial cells and targeted to plasma membrane protrusions. J Biol Chem. 2000;275:5512–5520. doi: 10.1074/jbc.275.8.5512. [DOI] [PubMed] [Google Scholar]
- 47.Pannequin J, Delaunay N, Buchert M, Surrel F, Bourgaux JF, Ryan J, Boireau S, Coelho J, Pelegrin A, Singh P, Shulkes A, Yim M, Baldwin GS, Pignodel C, Lambeau G, Jay P, Joubert D, Hollande F. Beta-catenin/Tcf-4 inhibition after progastrin targeting reduces growth and drives differentiation of intestinal tumors. Gastroenterology. 2007;133:1554–1568. doi: 10.1053/j.gastro.2007.08.023. [DOI] [PubMed] [Google Scholar]


