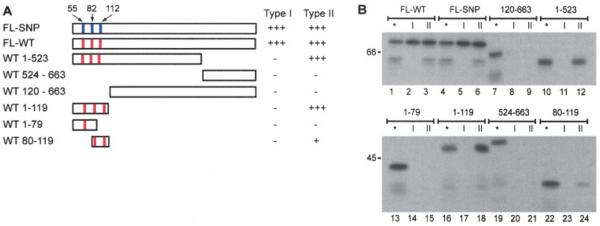
Figure 2.
Both subtypes of PAD-4 autoantibodies require the N-terminal domain encompassing the polymorphic residues for recognition. A, Schematic representation of the PAD-4 constructs used for the immunoprecipitation studies shown in B. Red vertical lines denote S55, A82, and A112 in wild-type (WT) PAD-4. Blue vertical lines denote G55, V82, and G112 in polymorphic PAD-4. The ability of type I and type II PAD-4 antibodies to immunoprecipitate the different constructs is summarized on the right. B, Equally labeled amounts (assessed by densitometry) of different 35S-methionine–labeled PAD-4 products were immunoprecipitated with representative type I or type II PAD-4 autoantibody–positive RA sera. All constructs in the lower panel were fusion proteins containing glutathione S-transferase (GST). None of the sera recognized GST alone (results not shown). For each of the different IVTT constructs, a gel sample consisting of one-fifth of the volume used for immunoprecipitation (lanes labeled with an asterisk) was electrophoresed adjacent to the paired immunoprecipitations. Type I sera recognized only full-length (FL) PAD-4 constructs, and removal of either the N- or C-terminal domains prevented recognition. Type II sera immunoprecipitated all constructs containing the 119 N-terminal amino acids of PAD-4. SNP = single-nucleotide polymorphism (see Figure 1 for other definitions).