Scheme 1. Synthesis of the Benzo[a]quinolizidine Subunita.
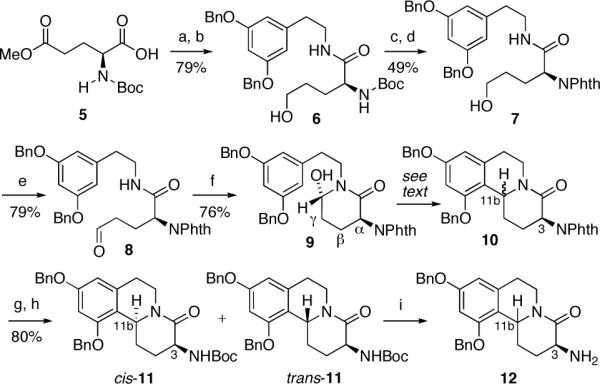
aConditions: (a) i-BuOCOCl, NMM, CH2Cl2, -35 °C, 15 min, then 2-[3,5-bis(benzyloxy)phenyl]ethylamine, DMF, CH2Cl2, rt, 12 h; (b) NaBH4, LiCl, THF, MeOH, 0 °C, 5.5 h; (c) HCl-Et2O, CH2Cl2, rt, 2 h; (d) PhthCO2Et, Na2CO3, THF, rt, 16 h; (e) Swern oxidation; (f) AcOH, CH2Cl2, rt, 2 h; (g) H2NNH2, EtOH, rt, 24 h; (h) Boc2(O), CH2Cl2, rt, 1 h, cis-11 (57%), trans-11 (26%); (i) TFA, CH2Cl2, 0 °C, 1 h cis-12 (C11b-Ha, 98%), trans-12 (C11b-Hb, 85%).
