Scheme 2. Total Synthesis of Schulzeine A (1).a.
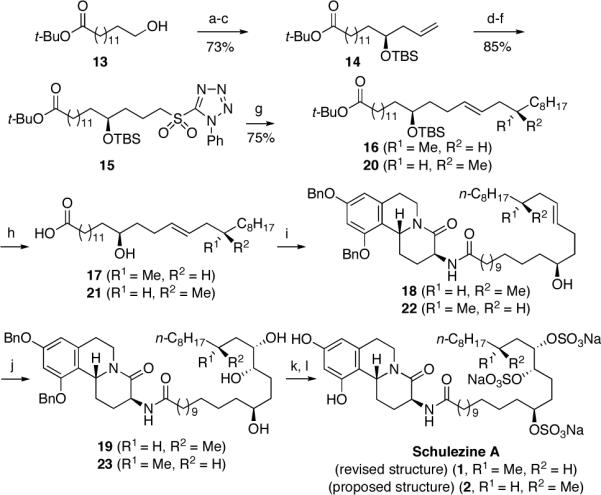
aConditions: (a) Moffat-Swern oxidation; (b) (R)-(+)-1,1'-bi-2-napthol (10 mol%), Ti(O-i-Pr)4, 4 Å m.s., CH2Cl2, reflux 1 h; then -78 °C, allyltri-n-butylstannane, -20 °C, 5 d; (c) TBSCl, imd, CH2Cl2, 0 °C®rt, 18 h; (d) 9-BBN, THF, 0 °C®rt, 14 h, then H2O2, NaOH, B(OH)3, rt °C, 1 h; (e) 1-phenyl-1H-tetrazole-5-thiol, DIAD, Ph3P, THF, 0 °C®rt, 12 h; (f) (NH4)6Mo7O24•4H2O (20 mol%), H2O2, EtOH, 0 °C®rt, 14 h; (g) KHMDS, DME, -60 °C, 30 min, then (S)-3-methylundecanal, -60 °C®rt, 16 h, 16, 45%; KHMDS, DME, -60 °C, 30 min, then (R)-3-methylundecanal, -60 °C®rt, 16 h, 20, 75%, d.r. > 20:1; (h) HF, TBAF, THF, rt, 48 h; NaOH (1 M), EtOH, THF, reflux, 48 h, 17, 70%; 21, 84%; (i) EDC, CH2Cl2, rt, 16 h, 18, 73%; 22, 80%; (j) AD-mix a, MeSO2NH2, t-BuOH-t-BuOMe-H2O-THF, 0 °C (1:1:1:1), 18 h, 19, 83%, d.r. = 4:1; AD-mix a, MeSO2NH2, t-BuOH-t-BuOMe-H2O (1:1:1), 0 °C, 48 h, 23, 77%, dr = 91:9; (k) SO3-pyr, DMF, rt, 2 h; (l) H2, Pd/C, EtOH, rt, 8 h, 4, 88% (two steps); 1, 75% (two steps).
