Summary
Oncogene-induced senescence functions to limit tumor development. However, a complete understanding of the signals that trigger this type of senescence is currently lacking. We found that mutations affecting NF1, Raf and Ras induce a global negative feedback response that potently suppresses Ras and/or its effectors. Moreover, these signals promote senescence by inhibiting the Ras/PI3K pathway, which can impact the senescence machinery through HDM2 and FOXO. This negative feedback program is regulated, in part, by RasGEFs, Sprouty proteins, RasGAPs, and MKPs. Moreover, these signals function in vivo, in benign human tumors. Thus, the ultimate response to the aberrant activation of the Ras pathway is a multi-faceted negative feedback signaling network that terminates the oncogenic signal, and participates in the senescence response.
Significance
While activation of the Ras pathway generally promotes tumorigenesis, in some benign tumors deregulated oncogenic signaling triggers cellular senescence. Consequently, “oncogene-induced senescence” (OIS) is thought to limit the progression of these lesions. However, the molecular mechanisms underlying OIS are not completely understood, and it is unclear why some cells senesce in response to oncogenic insult while others do not. We have found that “sensitive cells” trigger a negative feedback signaling network, designed to terminate aberrant signals. Moreover, this response actively promotes senescence and is mediated by numerous proteins known to regulate Ras signaling. Finally, this negative feedback program functions in benign human tumors. Thus, these studies provide mechanistic insight into OIS and reveal additional genes that function in tumor suppression.
Introduction
Aberrant activation of the Ras pathway plays an undisputed role in human cancer (Downward, 2003). However, in some primary cells activated Ras induces the accumulation of p53, p16 and ARF and triggers cellular senescence (Serrano et al., 1997). This apparent paradox can be reconciled by the observation that Ras-induced senescence is bypassed by inactivating Rb and p53, suggesting that this response may have evolved as a mechanism of tumor suppression (Serrano et al., 1997). Importantly, recent reports have further demonstrated that oncogene-induced senescence occurs in vivo in response to Ras, Raf and PTEN mutations in human tumors and in mouse tumor models (Braig et al., 2005; Chen et al., 2005; Collado et al., 2005; Michaloglou et al., 2005). In each case, a senescent population of cells was detected in benign but not advanced lesions, supporting the in vitro observation that activation of these pathways leads to an initial burst of proliferation followed by cellular senescence. Thus, these data suggest that senescence functions as a mechanism of tumor suppression by limiting the development of these benign lesions, in the absence of additional cooperating mutations.
However, while these studies support an important biological role for oncogene-induced senescence, the molecular mechanisms that trigger this response are not completely understood. Certainly some of the signals that contribute to the senescence response have been identified. For example, activation of the Ras/Raf pathway is known to mediate some of its effects through activation of p16 and ARF (Ohtani et al., 2001; Wei et al., 2001; Zhu et al., 1998). However, many additional questions remain unanswered. First, it appears that some cells are sensitive to oncogene-induced senescence while other cell-types are not (Collado et al., 2005; Tuveson et al., 2004), although there is no current mechanistic explanation for this observation. In addition, the historical definition of a senescent cell is one that cannot activate immediate early genes in response to growth factors (Seshadri and Campisi, 1990). This observation implies that there must be a signal(s) that actively suppresses early signal transduction events in senescent cells, which presumably contributes to their inability to proliferate. However this aspect of senescence cannot be intuitively explained by the known functions of p53 and Rb.
To address these questions we first developed a system in which we could study cell types that might respond differently to the same oncogenic insult. Specifically, we examined the consequences of inactivating the NF1 tumor suppressor. The NF1-encoded protein, neurofibromin (NF1), is a Ras GTPase activating protein (GAP), and has been shown to function as such in vitro and in vivo (reviewed in (Cichowski and Jacks, 2001)). Loss-of-function mutations in NF1 underlie the familial cancer syndrome neurofibromatosis type I, which is characterized by the development of a variety of tumorigenic and non-tumorigenic symptoms. Notably, many of the lesions associated with NF1 are benign. We and others have previously shown that Ras is hyper-activated in NF1-deficient mouse cells and human tumor cell lines (Basu et al., 1992; Bollag et al., 1996; Cichowski et al., 2003; DeClue et al., 1992; Johannessen et al., 2005; Kim et al., 1995). Accordingly, we reasoned that acutely inactivating neurofibromin, via an RNAi strategy, would provide a unique means of activating physiological levels of Ras in different cell lines, which cannot be accomplished by ectopically expressing an activated Ras protein.
Interestingly, loss of neurofibromin in primary mouse embryonic fibroblasts (MEFs) resulted in their immortalization, similar to the phenotype of MEFs expressing a single mutant K-ras allele (Tuveson et al., 2004). In contrast, acute neurofibromin-deficiency in primary human cells rapidly triggered senescence. Surprisingly, while Ras, AKT and ERK activity were sustained in MEFs upon neurofibromin loss, in primary human cells these proteins were transiently activated and then quickly suppressed to lower than baseline levels. This negative feedback response was not limited to NF1 knock-down (NF1KD) cells, as a mutant Raf allele also induced a rapid and dramatic suppression of Ras and the PI3K/AKT pathway. In both cases, this negative feedback response appeared to be mediated by several classes of proteins known to suppress Ras signaling. In addition, we found that the suppression of PI3K in this context can interface with Rb and p53 pathways minimally through its effects on HDM2 and FOXO, and does so in vivo in benign human tumors. Taken together, these data suggest that one mechanism by which a cell protects itself from oncogenic insult is by initiating a fail-safe negative feedback signaling program that ultimately promotes the senescence response. Thus, these negative regulatory proteins may be unappreciated components functioning to limit the development of benign tumors.
Results
Effects of NF1 loss on primary human and mouse cells
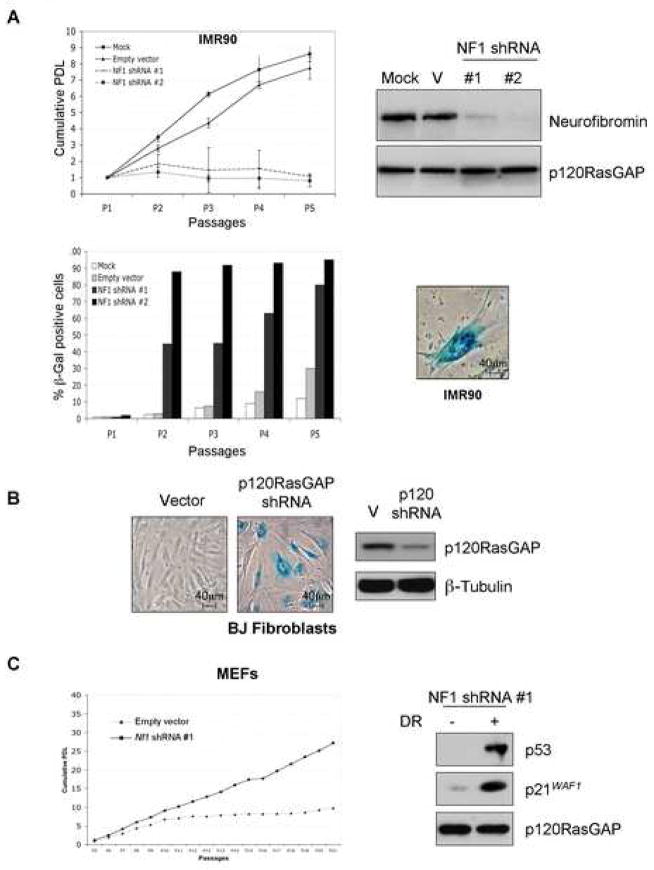
To investigate mechanisms that contribute to oncogene-induced senescence, the effects of neurofibromin-deficiency were compared in several cell systems in which Ras-mediated senescence has been well-studied (Serrano et al., 1997; Wei and Sedivy, 1999). Human IMR90 or BJ fibroblasts were infected with lentiviruses encoding shRNAs recognizing two different sequences within the NF1 gene. Both constructs suppressed neurofibromin expression and induced senescence within 5 days (Figure 1A). Both IMR90 and BJ fibroblasts became senescent with roughly the same kinetics, as determined by an observed growth arrest, senescence-associated β-galactosidase activity (SA-β-gal), and a flattened cellular morphology (Figure 1Aand data not shown). Notably, p120RasGAP shRNAs also triggered cellular senescence, supporting the conclusion that senescence in these cells was triggered by endogenous Ras activation, and was not due to an uncharacterized function of neurofibromin (Figure 1B).
Figure 1.
Loss of neurofibromin induces senescence in human fibroblasts, but immortalizes mouse embryonic fibroblasts
A: Growth curve and immunoblot of normal human IMR90 fibroblasts infected with a control lentiral vector (V), or lentiviruses expressing NF1 shRNAs. At each cell passage cells were re-plated and SA-β-gal activity and morphology was assessed (bottom panels).
B: SA-β-gal activity in response to p120RasGAP knock-down.
C: Proliferative properties of wild-type and NF1KD MEFs. Doxorubicin (DR) treatment (0.2 μg/ml for 18 hours) was used at passage 21, to assess p53 status.
Because physiological levels of oncogenic Ras have been reported to immortalize murine fibroblasts, rather than induce senescence (Guerra et al., 2003; Tuveson et al., 2004), we also assessed the biological effects of neurofibromin-deficiency in primary MEFs. Consistent with the phenotype conferred by an activated K-ras allele, Nf1-deficient MEFs did not undergo premature senescence but were immortal. This phenotype was observed in cells where neurofibromin expression was abolished through the use of the same shRNA-encoding construct used in primary human cells, and in several independently-derived Nf1-deficient MEF lines (Figure 1C and Supplementary Figure 1A). Immortalized Nf1-deficient and NF1KD MEFs maintained an intact p53 response to doxorubicin and retained ARF expression (Supplementary Figure 1B), indicating that these cells did not spontaneously immortalize by acquiring defects in these pathways. These results demonstrate that the same genetic event can trigger dramatically different biological responses in primary mouse cells (MEFs) versus primary human fibroblasts. While species-specific differences may contribute to these observed effects, based on additional findings described below, we believe that this observation reflects different biochemical responses to this aberrant signal in these particular cell types.
p53 and Rb pathways mediate senescence triggered by the loss of NF1
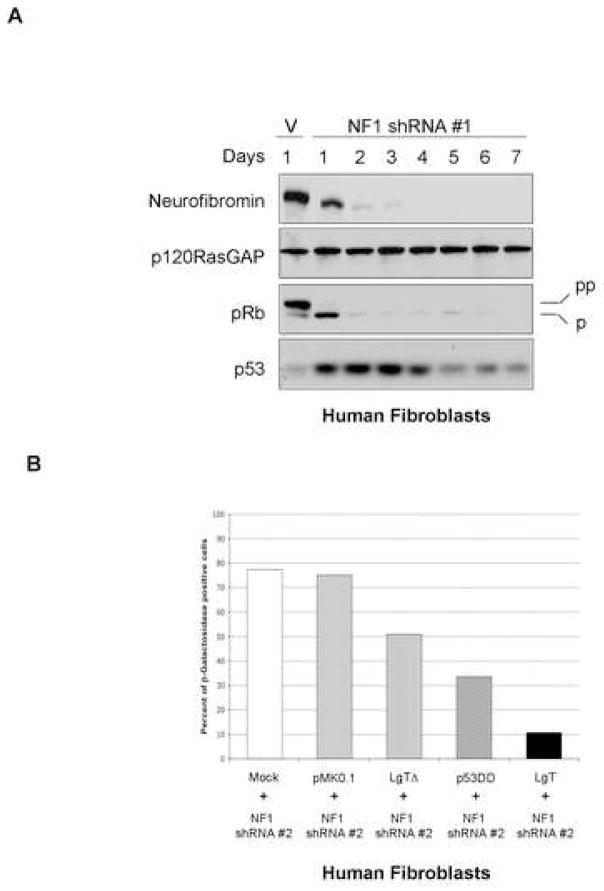
Senescence in response to Ras activation in human cells is mediated by the activation of the Rb and p53 pathways (Serrano et al., 1997), therefore the expression of these proteins was examined in NF1KD cells. Similar to what has been reported in cells ectopically expressing an activated Ras allele, loss of neurofibromin induced the accumulation of p53 and the activation of Rb, as demonstrated by reduced levels of hyper-phosphorylated Rb (Figure 2A). To assess the relative contribution of these pathways to the senescence response, Rb and p53 pathways were inactivated in isolation or together. The p53 pathway was disrupted by expressing a dominant-negative p53, and the Rb pathway was inactivated by expressing an SV40 Large T mutant, capable of binding and inactivating all 3 Rb family members, but deficient in its ability to bind p53 (Hahn et al., 2002). Inactivation of either the Rb or p53 pathway resulted in a partial decrease in the percentage of senescent cells, but in isolation, were unable to completely override the effects of NF1 inactivation (Figure 2B). However, senescence was dramatically inhibited in cells expressing the wild-type SV40 Large T antigen, which binds and inactivates Rb family members and p53. These results indicate that both the Rb and p53 pathways mediate NF1-related senescence in human cells.
Figure 2.
Senescence triggered by neurofibromin-deficiency requires Rb and p53 pathways
A: Immunoblots demonstrating that p53 and Rb are activated in response to NF1-loss.
B: NF1 was inactivated in cells previously infected with an empty vector (pMKO.1), LgT, a dominant-negative p53 (p53DD), or a Large T Δ234-444 mutant. SA-β-gal-expression is shown.
Ras and Ras effector pathways are rapidly inactivated in human cells following oncogenic insult
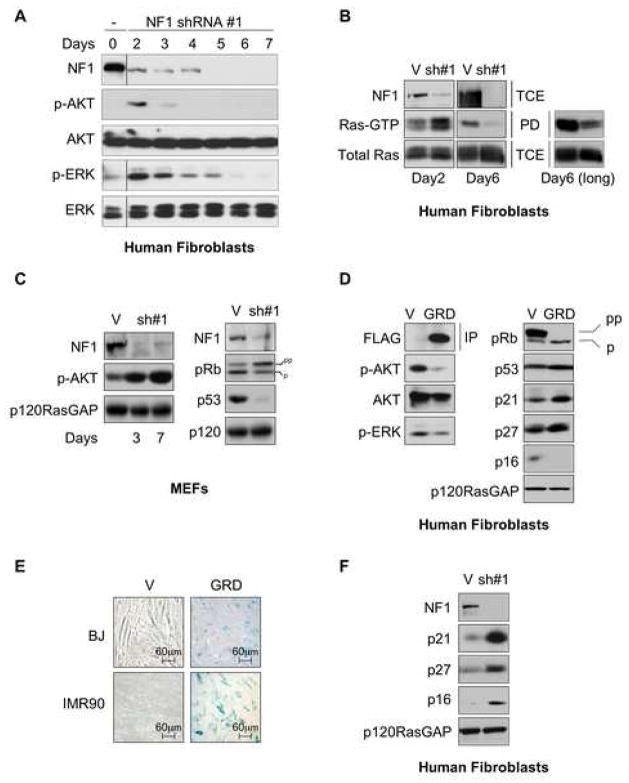
To examine the effects of neurofibromin loss on cell signaling, the activation of ERK and AKT were assessed in human cells following exposure to NF1-specific, shRNA-expressing lentiviruses. Neurofibromin expression was significantly decreased within 2 days after infection (Figure 3A). Concomitant with this decrease was an increase in ERK and AKT activity as assessed by their phosphorylation status. Surprisingly however, the activation of these proteins peaked at day 2 and began to decrease rapidly thereafter. Phospho-AKT and phospho-ERK levels were potently suppressed by days 3 and 4, respectively. Ras-GTP levels exhibited similar kinetics: Ras-GTP levels were elevated 2 days after infection and were suppressed to levels significantly lower than cycling vector infected cells several days later (Figure 3B). Phospho-AKT levels were similarly reduced to levels below baseline (Supplementary Figure 2). Importantly, at both time points cells were plated at an equal density, eliminating any potentially confounding effects of cell density in these studies. Thus, while the loss of neurofibromin initially triggers the activation of Ras and its effectors in primary human cells, these pathways are potently suppressed shortly thereafter.
Figure 3.
Negative feedback suppression of the Ras pathway underlies cellular senescence
A: Immunoblots of IMR90 lysates showing the dynamic effects of NF1-loss on pERK and pAKT levels. Intervening lanes are spliced out where indicated, but all time-courses represent data from the same immunoblot.
B: Ras activation in NF1KD versus control cells on days 2 and 6 (see Experimental procedures). The long exposure is shown to highlight the decrease in Ras-GTP levels between vector and NF1 knock-down cells at this latter time point.
C: pAKT, Rb and p53 immunoblots of MEF lysates in response to NF1-deficiency.
D: Immunoblots of lysates from BJ fibroblasts infected with retrovirus expressing the GRD of neurofibromin, 9 days post-infection.
E: SA-β-gal staining of fibroblasts (IMR90 and BJ) infected with the GRD of neurofibromin.
F: Immunoblots of cell lysates from BJ fibroblasts in response to NF1-loss, 6 days post-selection.
These observations were in stark contrast to the effects of neurofibromin loss in MEFs, which did not senesce but rather became immortalized (Figure 1C and Supplementary Figure 1). Notably, we and others have extensively demonstrated that NF1-deficiency in MEFs results in a sustained activation of Ras and Ras-effector pathways ((Cichowski et al., 2003; Hiatt et al., 2000; Johannessen et al., 2005) and Figure 3C). Moreover, we found that Rb and p53 were not activated in response to the acute loss of NF1 (Figure 3C). We do not believe that these observations are due to inherent species-specific differences between mouse and human cells, as mouse cells do undergo senescence in response to oncogenic insult in vivo (Chen et al., 2005; Collado et al., 2005). Moreover, Nf1-deficiency results in a growth arrest of murine Schwann cells in culture (Kim et al., 1995). Therefore, we favor the possibility that the differential biochemical and biological responses to aberrant Ras activation, represent differences in the signaling network components that are present in these particular cell types. These results also support the possibility that traditional signals that are triggered by this genetic event may not be sufficient to induce senescence alone, and that additional cooperating signals may be required to amplify this response in MEFs.
Suppression of the Ras pathway is sufficient to trigger cellular senescence
Notably, in IMR90 and BJ cells, Ras signaling was suppressed prior to the initiation of senescence. To examine whether this suppression might actively contribute to the senescence response, IMR90 and BJ fibroblasts were infected with a retrovirus that expresses the catalytic GAP-related domain (GRD) of neurofibromin. Expression of this domain has previously been shown to decrease Ras-GTP levels (Hiatt et al., 2000; Johannessen et al., 2005). Consistent with these findings, GRD expression resulted in a significant decrease in the activation of AKT and ERK (Figure 3D), and induced senescence in both IMR90 and BJ cells (Figure 3E), suggesting that suppression of Ras is sufficient to trigger cellular senescence.
Importantly, GRD expression resulted in the activation of both the Rb and p53 pathways (Figure 3D), and the same cell cycle inhibitors were up-regulated in human cells treated with NF1-specific shRNA constructs (Figure 3F), with the exception of p16, which was only activated in NF1KD and not GRD-expressing cells. This latter finding was predicted, as activation of ERK increases p16 expression via its effects on ETS transcription factors (Ohtani et al., 2001), which would only be expected to happen in response to neurofibromin loss. In addition, p16-expression becomes Raf-independent in cells expressing an inducible Raf allele, once p16 is up-regulated (Zhu et al., 1998). Nevertheless, the observation that 1) the Ras signal is terminated prior to senescence in NF1-deficient cells, that 2) Ras suppression is sufficient to trigger senescence, and that 3) Ras inactivation results in the activation of both the Rb and p53 pathways, suggests that this event actively participates in the senescence response and may do so by cooperating with or amplifying previously described signals. In this context it is notable that the suppression of the PI3K/AKT pathway in particular, is coincident with the induction of p16 in response to acute NF1-deficiency (Supplementary Figure 3). Therefore this signal is poised to function along with p16 in mediating this process. The significance of PI3K/AKT suppression will be addressed in Figure 5.
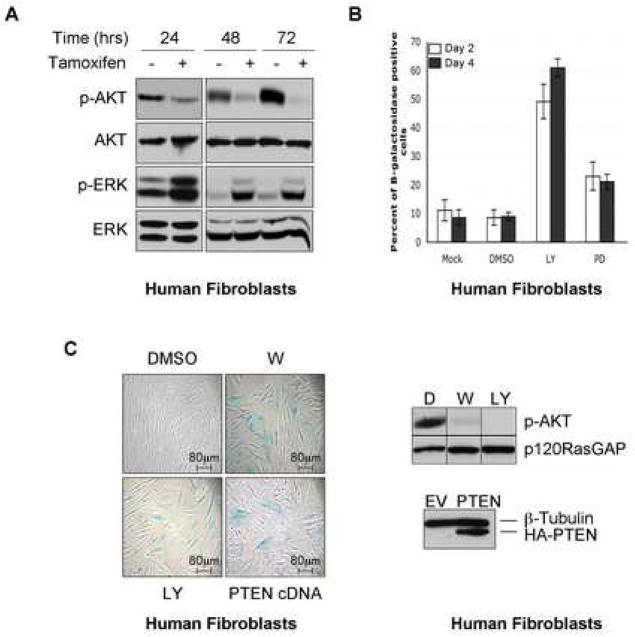
Figure 5.
Inactivation of the PI3K pathway triggers senescence
A: BJ fibroblasts, expressing an inducible form of Raf, were treated with 1 μM tamoxifen (+) or ethanol (−). Immunoblots demonstrate the dramatic decrease in pAKT levels.
B: Percentage of SA-β-gal positive IMR90 cells after mock, DMSO, LY294002 and PD98059 treatments lasting 2 or 4 days.
C: SA-β-gal staining of BJ cells treated with DMSO, LY294002, Wortmannin or expressing an HA-PTEN (left). Control immunoblots are shown (right).
The aberrant activation of Raf and Ras trigger a global negative feedback signaling program
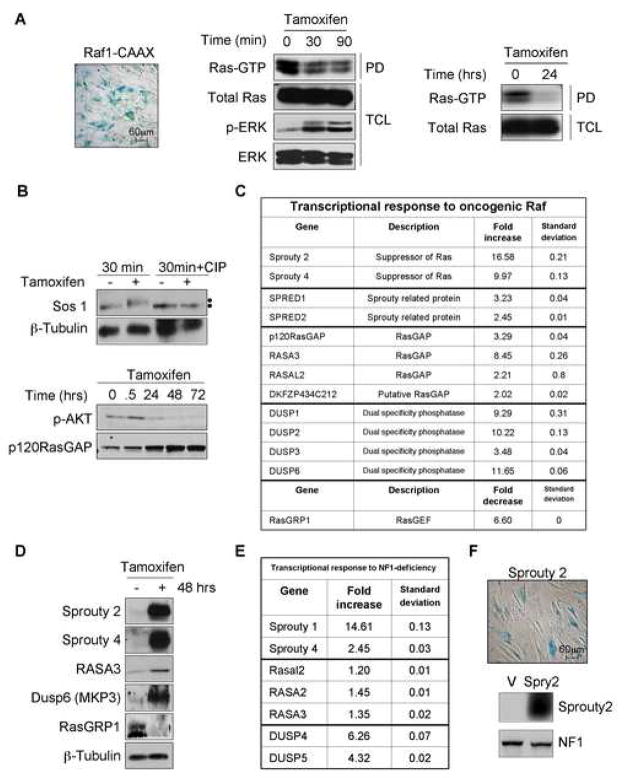
To determine whether the suppression of Ras and/or Ras effector pathways is a common mechanism leading to senescence, we looked for evidence of a negative feedback signal in response to Raf activation, which also triggers the senescence of primary human cells ((Zhu et al., 1998) and Figure 4A). When an inducible, activated Raf allele was expressed in BJ cells, Ras-GTP levels were significantly reduced within 30 minutes (Figure 4A). Strikingly, Ras-GTP levels were nearly abolished 24 hours later, suggesting the possible involvement of both short and longer-term mechanisms of suppression.
Figure 4.
Aberrant Raf activation suppresses Ras via multiple distinct mechanisms
A: SA-β-gal staining of IMR90 infected with Raf1-CAAX (left panel). BJ fibroblasts, expressing an inducible Raf construct, were treated for 30 or 90 minutes with tamoxifen (4-HT, 1 μM). Ras-GTP levels were assessed using a Ras pull-down (PD) assay. Control immunoblots on total cell lysates (TCL) are shown (middle panel). Ras-GTP levels after 24 hours of 4-HT (right panel).
B: SOS immunoblots of BJ cells expressing the inducible Raf construct in the presence and absence of CIP (added to lysates) (top). p120RasGAP protein levels increase as pAKT levels decrease (bottom).
C: BJ cells expressing the inducible Raf were treated for 42 hours with tamoxifen (1 μM) or ethanol. Real-time PCR was performed in triplicate on the listed genes and fold changes were calculated.
D: Immunoblot analysis of lysates from C.
E: Real-time PCR analysis of genes in response to NF1-loss, as compared to cycling, vector-expressing cells.
F: SA-β-gal staining and immunoblots of BJ cells expressing exogenous Sprouty 2.
To dissect the molecular events underlying this negative feedback pathway, we first examined the effects of Raf activation on known Ras regulators: RasGEFs and RasGAPs. Notably, the exchange factor SOS can be phosphorylated by ERK, which has been shown to result in its inactivation (Corbalan-Garcia et al., 1996; Dong et al., 1996). We found that SOS became phosphorylated in this system within 30 minutes of Raf activation, as demonstrated by a shift in its electrophoretic mobility, and confirmed by calf instestinal phosphatase (CIP) treatment, which eliminated the slower migrating species (Figure 4B). In addition, Raf significantly increased the protein levels of p120RasGAP within 24 hours to 72 hours (Figure 4B). These observations suggested that multiple signals, involving post-translational and perhaps transcriptional events, might be involved in triggering this negative feedback loop.
To obtain a more comprehensive view of the signals participating in this response, a preliminary microarray screen was performed on Raf-expressing cells as a means of identifying potential candidate genes. From this list of differentially regulated genes, those known to regulate Ras signaling were selected for real-time PCR and Western analysis (Figure 4C and 4D). Strikingly, several classes of genes, encoding proteins known to suppress Ras signaling at many levels, were shown to be exquisitely sensitive to sustained Raf activation. Among these candidates, Sprouty genes (2 and 4) were the most highly up-regulated in response to Raf activation. Sprouty was originally identified in a Drosophila screen as a suppressor of FGF receptor signaling, and has subsequently been shown to suppress the Ras pathway, potentially acting at many levels (reviewed in (Kim and Bar-Sagi, 2004)). In addition, four RasGAP-encoding genes, including p120RasGAP, were transcriptionally up-regulated in response to Raf activation, which would also be expected to directly suppress Ras-GTP levels. RasGRP1, which encodes a Ras exchange factor, was also down-regulated by more than 6-fold in response to Raf activation. Thus, Raf activation appears to suppress Ras-GTP levels directly through a variety of redundant mechanisms, although because of this redundancy we were not able to experimentally determine which of these events are absolutely critical in this system. Interestingly, other suppressors of Ras signaling known to act on downstream components, were also identified. The Sprouty-related genes, Spred-2 and -4, were also highly up-regulated, and have been shown to suppress Raf signaling, by affecting the Ras/Raf interaction (reviewed in (Kim and Bar-Sagi, 2004)). Finally, several dual specificity kinases (DUSPs), also referred to as MAP kinase phosphatases, known to inactivate ERK, were transcriptionally up-regulated in response to Raf, consistent with a previous study that identified genes that are up-regulated in response to Ras mutations in mouse and human tumors (Sweet-Cordero et al., 2005). Importantly, the loss of neurofibromin also triggered the up-regulation of Sprouty genes, DUSP genes, and a modest activation of RasGAP-encoding genes (Figure 4E). The up-regulation of these genes was overall more subtle in response to NF1-loss and interestingly, while the same classes of genes were affected, the specific genes identified were different. These differences may reflect differences in signal intensity and/or the coordinate effects of activating both the Raf and PI3 kinase pathway in NF1-deficient cells.
These data indicate that one of the earliest responses to the aberrant activation of the Ras pathway is the initiation of a negative feedback signaling network, designed to terminate the resulting oncogenic signal. Given that our analysis was primarily designed to detect differences in gene and protein expression, it is likely that these findings are an under-representation of the signals that may function in this context, and that more may occur via post-translational mechanisms. Moreover, because of this redundancy, loss of any one of these signals alone would not be expected to prevent senescence in this system, especially given that the inactivation of p53 or Rb alone are unable to bypass this response. However, we found that senescence could be triggered by recapitulating some of these signals. For example, ectopic expression of Sprouty 2 was sufficient to trigger a senescence response (Figure 4F). In addition, an increase in total cellular GAP activity, via the ectopic expression of a catalytic GAP domain, induced senescence as shown in Figure 3E. Taken together, these data further support the involvement of these signals in promoting oncogene-induced senescence.
In the analysis described above, several genes were identified that encode proteins known to affect Ras-GTP levels directly. However, some of these signals would not be expected to impact mutant Ras alleles. To determine if ERK and AKT were similarly suppressed in response to a mutant Ras allele at later time-points, the kinetics of ERK and AKT activation in human cells infected with a RasV12-expressing retrovirus were examined. Shortly after Ras was introduced, AKT and ERK were potently activated, however ERK and AKT were dramatically inhibited shortly thereafter (Supplementary Figure 4). While we did not attempt to identify the specific mediators of this response, these results further support the notion that the ultimate response of primary human cells confronted with the aberrant activation of the Ras pathway induced by different genetic events, is to dramatically attenuate Ras signaling, which can occur at many levels.
Suppression of the PI3K pathway mediates the senescence response
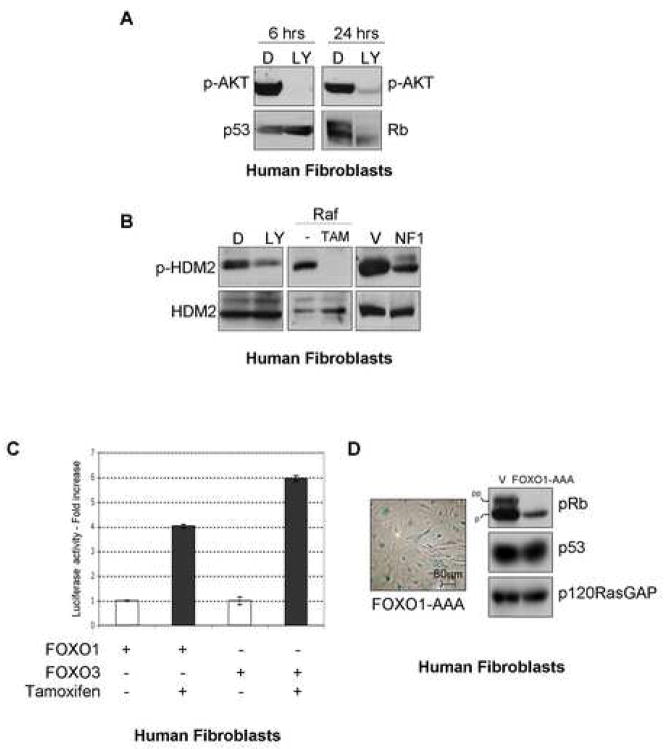
Because the suppression of Ras triggered cellular senescence, suppression of known effector pathways might be expected to mediate this effect. Interestingly, in response to an inducible Raf allele, the PI3K/AKT pathway was completely suppressed within 48–72 hours in BJ cells, while ERK activity was reduced but was never completely inactivated (Figure 5A). This observation, along with the delayed suppression of ERK relative to AKT in NF1KD cells, suggested that suppression of the PI3K pathway might play a greater role in mediating senescence. To investigate the relative contributions of these pathways, wild-type IMR90 cells were treated with the PI3K inhibitor LY294002 or the MEK inhibitor PD98059. In less than 3 days, 60 % of the cells treated with the PI3K inhibitor became senescent (Figure 5B, 5C and data not shown), as has been previously reported (Tresini et al., 1998). The PI3K inhibitor, wortmannin, and the ectopic expression of PTEN, which inactivates PI3K signaling, induced the same response (Figure 5C). In contrast, the MEK inhibitor only slightly increased the number of SA β-gal expressing cells. Therefore we conclude that suppression of the PI3K pathway is likely to be the primary mechanism by which this negative feedback loop promotes senescence.
Importantly, PI3K inhibition resulted in the activation of Rb and p53 demonstrating that the suppression of this pathway can impact these known senescence regulators (Figure 6A). To define the mechanism by which this occurred, we first examined pathways that are known to be affected by PI3K/AKT signaling. Notably, MDM2 phosphorylation by AKT at serine 166 (and 186) has been reported to activate its ubiquitin ligase activity toward p53 (Ogawara et al., 2002). Accordingly, HDM2 phosphorylated at serine 166 was readily detected in cycling BJ cells (Figure 6B). However, HDM2 phosphorylation levels were significantly reduced in cells treated with a PI3K inhibitor, and were reduced to an even greater extent in cells exposed to an activated Raf allele for 48 hours, or in NF1KD cells. Thus, this represents one mechanism by which suppression of AKT may transduce its effects on p53 in these cells.
Figure 6.
Oncogenic insult regulates HDM2 and FOXO proteins
A: BJ fibroblasts were treated for the indicated times with LY294002. Rb, p53 and p-AKT levels were assessed by immunoblot.
B: Levels of p-HDM2 were assessed by immunobloting lysates from BJ cells treated with DMSO (D) or LY294002 (LY) for 24 hours, from cells expressing an inducible Raf construct 48 hours after induction, or in NF1KD cells after 6 days.
C: Luciferase activity of a FOXO reporter in the presence of exogenous FOXO1 or FOXO3, 72 hours after Raf induction in BJ cells.
D: A phosphorylation mutant of FOXO1 (FKHR-AAA) induces senescence and Rb activation in human fibroblasts.
Because one of the most well-characterized events that occurs in response to suppression of the PI3K pathway is the activation of FOXO proteins, we also examined this pathway in this system. Active FOXO proteins are known to induce a growth arrest, and have been hypothesized to be tumor suppressors (reviewed in (Greer and Brunet, 2005)). Strikingly, Raf activation triggered the activation of FOXO proteins with the same rapid kinetics (48 hours) observed for the suppression of PI3K/AKT (Figure 6C). Moreover, the expression of a constitutively activated FOXO1 mutant, incapable of being inactivated by AKT, rapidly triggered cellular senescence along with the activation of Rb, but not p53 (Figure 6D). While we did not extensively characterize the expression of FOXO targets in these cells, we did confirm that BTG1, a FOXO target implicated in growth arrest, was transcriptionally up-regulated in Raf expressing cells within 48 hours by 4.25 fold (+/−.06) (Supplementary Figure 5). Thus, taken together these results suggest that the dramatic suppression of the PI3K/AKT pathway, in the context of Raf and NF1 mutations, may promote senescence, in part, through the inhibition of HDM2 activity and activation of FOXO proteins. These proteins may or may not be the only mediators of this process, and the specific signals may differ between cell-types. Nevertheless, these results indicate that the suppression of PI3K can impact the senescence response on many levels, and therefore is an unappreciated and multi-faceted signal that is engaged in response to the aberrant activation of Ras and Raf.
Senescence in benign human tumors from NF1 patients
To assess the in vivo relevance of this negative feedback program we examined tumors from patients with neurofibromatosis type 1. Specifically, benign peripheral nervous system tumors, known as neurofibromas, were examined. While these tumors are benign, a subset of lesions, plexiform neurofibromas, can progress to malignancy (Riccardi, 1992). However, for this study we focused on dermal neurofibromas, which are typically very small and never become malignant. One of the unusual features of neurofibromas is their cellular heterogeneity: consisting of Schwann cells, fibroblasts, perineurial cells, neurons, and mast cells (reviewed in (Cichowski and Jacks, 2001). Human genetic studies and mouse modeling efforts indicate that neurofibroma development is triggered by a somatic second-hit mutation in the NF1 gene, that occurs in a Schwann cell progenitor (Zhu et al., 2002). However, while these NF1−/− cells serve as a seed population to initiate tumorigenesis, surrounding cells are recruited in the process of tumor development. As a consequence, a high percentage of cells distributed throughout neurofibromas are merely heterozygous for an NF1 mutation.
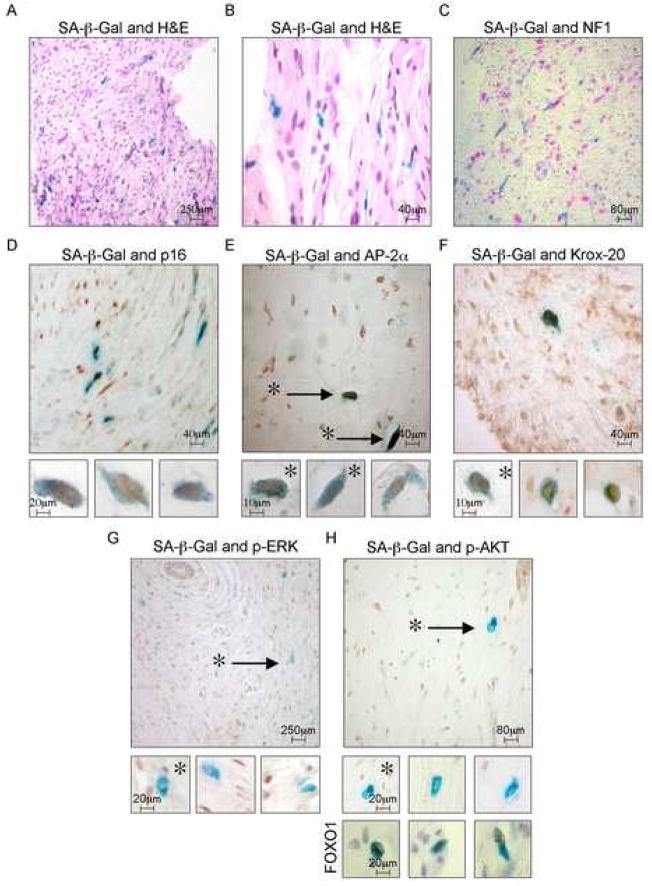
Notably, SA-β-gal expressing cells were observed in 5 out of 5 dermal neurofibromas isolated from NF1 patients (Figure 7A and B). Given the cellular complexity of these lesions, which are comprised of numerous NF1 +/− cells as described above, the dispersed pattern of SA-β-gal expressing cells was expected. In addition, tumors are often resected when they are symptomatic and growing, and therefore would not be predicted to be completely “senescent”. Regardless, while the percentage of SA-β-gal cells was small (ranging from 2.7 – 5.1 % in 5 tumors), SA-β-gal activity was never found in cells that retained neurofibromin expression (NF1 +/− cells), further indicating that senescence is triggered by NF1-deficiency (Figure 7C).
Figure 7.
Senescence occurs in human neurofibromas in vivo
A and B: SA-β-gal expression in dermal neurofibromas.
C: A bright-field picture of a SA-β-gal stained section (blue) overlayed with a dark-field image of immunofluorescent neurofibromin staining (pink). Dermal neurofibroma sections, first stained for SA-β-gal, were then stained for: D: p16, E: AP2-α, F: Krox-20. G: p-ERK, H: p-AKT (top large image and middle small images), and FOXO1 (bottom small images). High magnification images of single cells demonstrate the nuclear localization of FOXO1 and an absence of nuclear p-ERK or p-AKT staining in all senescent cells. For FOXO1 analysis sections were counterstained with hematoxylin to demonstrate surrounding negative cells.
To determine whether these SA-β-gal expressing cells exhibited additional markers of senescence, immunohistochemistry was performed using antibodies to p16 (Figure 7D). Notably, 100% percent of the SA-β-gal expressing cells expressed p16, as assessed by examining over 300 cells from 5 individual tumors. However, in all cases there was a higher percentage of p16-expressing cells than SA-β-gal expressing cells (8.1 % versus 5.1 % in this tumor), similar to observations in benign melanocytic nevi, further supporting the hypothesis that p16 expression on its own may not be sufficient to induce senescence in these cells and additional signals could be required (Michaloglou et al., 2005).
Senescence occurs in a progenitor cell population
If senescence limits the development of these lesions, it would be predicted to occur in Schwann cell progenitors, as this lineage drives tumor development. Therefore, nuclear markers of Schwann cell progenitors were examined: specifically AP2-α and Krox-20. AP2-α is a transcription factor that is expressed in neural crest stem cells and its expression is terminated as Schwann cell precursors differentiate into embryonic Schwann cells (Stewart et al., 2001). Krox-20 is expressed at a slightly later stage of Schwann cell development (Ghislain et al., 2002), but importantly mice carrying a mutant floxed Nf1 allele develop neurofibromas when crossed to a Krox-20 Cre expressing mouse strain, indicating that Krox-20-expressing cells can serve as a progenitor population capable of driving tumor development (Zhu et al., 2002). Interestingly, the majority of SA-β-gal-expressing cells expressed AP2-α and Krox-20. However, like p16, not all AP2-α or Krox-20-expressing cells expressed SA-β-gal (Figure 7E and 7F), indicating that not all of these cells were undergoing senescence at the time the tumor was removed. Intriguingly, these results suggest that senescence occurs in a Schwann cell progenitor population within human neurofibromas.
Negative feedback signals occur in senescent cells in human tumors
Our in vitro data indicated that the inactivation of the Ras pathway participates in triggering senescence in response to NF1-deficiency. Activated ERK and AKT were readily detected in the nuclei of a subset of cells scattered throughout the tumors; however, p-ERK and p-AKT were never detected in the nuclei of senescent cells, as determined by examining over 300 cells from 5 individual tumors (Figure 7G and H). We also examined FOXO1, a known target of AKT, that is exported to the cytoplasm upon phosphorylation, where it can be degraded (Greer and Brunet, 2005). Notably. FOXO1 was exclusively detected in the nuclei of senescent cells, further supporting the conclusion that AKT is inactivated in these cells and that this signal results in the activation of FOXO proteins (Figure 7H). Collectively, these results indicate that the negative feedback response that we dissected in vitro, also occurs in vivo, specifically within a senescent population of cells within benign tumors. These data suggest that this negative feedback loop coordinately functions with previously identified signals to mediate oncogene-induced senescence.
Discussion
The cellular mechanisms that suppress tumorigenesis have been investigated for many years. However, while apoptosis is a widely accepted mechanism of tumor suppression, oncogene-induced senescence has been more controversial. Nevertheless, a series of recent papers have demonstrated that Ras, Raf and PTEN mutations trigger senescence in vivo in human and mouse tumors (Braig et al., 2005; Chen et al., 2005; Collado et al., 2005; Michaloglou et al., 2005). Moreover, these studies demonstrated that senescence exclusively occurs in benign tumors, indicating that it may function to prevent the progression of these lesions. However, the precise mechanisms that mediate oncogene-induced senescence have not been fully elucidated.
A revised model of oncogene-induced senescence
In this study, we show that the aberrant activation of endogenous Ras can induce dramatically different phenotypes in different cell types. In MEFs, loss of the NF1 tumor suppressor results in sustained Ras activation, a hypersensitivity to growth factors, and immortalization. However in normal human diploid fibroblasts loss of NF1 triggers a transient activation of Ras and Ras effectors, followed by a dramatic suppression of these signals to lower than baseline levels. Moreover these cells are not immortalized, but rather become senescent. Our results indicate that in “sensitive” cell types, the ultimate response to the aberrant activation of the Ras pathway is a dramatic termination of Ras signaling at many levels, followed by a cellular response designed to eliminate the proliferative potential of these cells. Furthermore, this sensitivity is not determined by a single biochemical event, but rather by the coordinated output of cell type-specific signaling networks.
The central conclusion from this study is that the consequential suppression of Ras and/or PI3K is an unappreciated signal that contributes to oncogene-induced senescence (Figure 8). Several observations support this conclusion. First, Ras effector pathways are rapidly extinguished in response to NF1 loss, and Raf and Ras mutations, prior to the onset of senescence. In addition, suppression of Ras and/or PI3K are sufficient to induce senescence, and these events on their own can activate the known downstream mediators of the senescence response (Rb and p53) through a variety of mechanisms. Prior to this study, oncogene-induced senescence was thought to be primarily mediated by p16 and ARF. However, recent studies support the involvement of additional uncharacterized signals in the senescence response. For example, in primary melanocytes an activated Raf allele induced p16 expression but did not induce ARF (Michaloglou et al., 2005). Nevertheless, the ablation of p16 had no effect on senescence induced by this oncogenic Raf allele. A similar anomaly was observed in vivo, as melanocytic nevi from p16-deficient Leiden patients, albeit larger, still underwent a growth arrest (Gruis et al., 1995). Therefore it has been suggested that there are additional signals that collaborate with p16 to promote or establish senescence (Michaloglou et al., 2005). Our data suggest that the negative feedback suppression of the Ras/PI3K pathway, and its downstream consequences (inhibition of HDM2 and activation of FOXO proteins, among others), represent such signals. Thus we propose a model whereby an oncogenic insult can trigger multiple inhibitory signals that may vary, depending on the genetic event and the specific signaling network components that are present in a particular cell-type. In cells with intact p53 and Rb pathways, we propose that these signals cooperate to achieve a threshold response, thereby inducing cellular senescence.
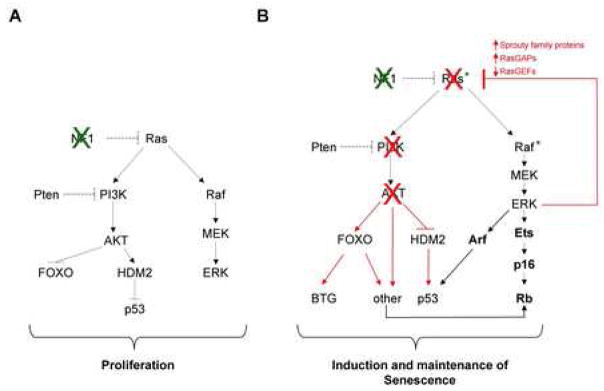
Figure 8.
Revised model of oncogene-induced senescence
A: In cells that are insensitive to oncogene-induced senescence, activation of the Ras pathway leads to hyper-activation of well known effector pathways which function to promote tumorigenesis.
B: In sensitive cells the ultimate response to the aberrant activation of the Ras pathway is the initiation of a multi-faceted negative feedback signaling network designed to terminate the oncogenic signal. These signals are triggered by the Raf/MEK/ERK pathway and involve numerous transcriptional and post-translational events, including the suppression of Ras exchange factors, and the up-regulation of Sprouty proteins and RasGAPs, among others. These inhibitory signals, via the consequential suppression of PI3K, can activate Rb and p53 through multiple signals. Thus, we propose that oncogene-induced senescence is mediated by this negative feedback signaling program, that functions along with other known senescence regulators, such as p16 and ARF, to achieve a threshold senescence signal.
The existence of multiple negative feedback pathways designed to inhibit Ras signaling makes teleological sense, in that it would be advantageous to immediately terminate the effects of aberrant Ras signaling at many levels. Notably however, in response to Raf mutations and NF1-loss, many of these negative feedback signals are directed toward Ras itself. Minimally, this regulation is likely to involve 3 families of proteins: RasGAPs, RasGEFs and Sprouty proteins. It is particularly notable that Sprouty genes were highly expressed in both Raf-expressing and NF1-deficient cells. Interestingly, a recent microarray study identified a Sprouty gene as one of the genes differentially up-regulated in benign human neurofibromas as compared to malignant tumors (Holtkamp et al., 2004), further supporting the hypothesis that this class of genes may be involved in limiting the development of these benign lesions. In any case, it is tempting to speculate that these negative regulatory proteins may be unappreciated tumor suppressors, and may ultimately be shown to be mutational targets in cancer.
PI3K and its role in senescence
Our data indicate that the suppression of PI3K, in particular, mediates the senescence response. These findings explain the longstanding observation that MEFs lacking the PI3K p85α and β subunits cannot be generated, as they undergo premature senescence (Brachmann et al., 2005). In addition, the observation that aberrant Raf activation potently suppresses PI3K signaling provides one mechanistic explanation for the known cooperativity of Raf and PTEN mutations in human tumors (Tsao et al., 2004). Interestingly, we found that Raf not only suppressed PI3K signaling, but as a consequence stimulated FOXO activity, revealing an additional unexpected connection between these two pathways. Notably, FOXO genes have been hypothesized to function as tumor suppressors (Greer and Brunet, 2005). Moreover, we found that the expression of a constitutively activated FOXO protein rapidly triggered senescence. Taken together, these data raise the intriguing possibility that FOXO proteins may specifically function in tumors harboring activated Raf alleles. Nevertheless, the consequential suppression of PI3K in response to Raf or NF1 mutations, may mediate its effects via a number of mechanisms, involving FOXO, HDM2, or other signals. Regardless, the observation that multiple negative feedback pathways are activated in this context, underscores the potential importance of these events in tumor suppression.
On the surface, this model appears to contradict recent findings indicating that the activation of PI3K, through loss of PTEN, also triggers senescence (Chen et al., 2005). We also found that loss of PTEN induced senescence in human cells, but we found no evidence of Ras suppression or a decrease in pAKT levels (unpublished observations). However, these data do not exclude the possibility that a negative feedback event may occur somewhere downstream of AKT. Regardless, these findings indicate that PI3K may be a critical sensor and regulator of the senescence response. Too little or too much PI3K activity can trigger senescence, the former mediated by the mechanisms described above, the latter perhaps resulting from the induction of ARF and p53, as has been previously suggested (Chen et al., 2005). Nevertheless, in the context of Raf mutations and NF1-deficiency (in vitro and in vivo), negative feedback signals designed to suppress PI3K signaling appear to predominate; the PI3K/AKT pathway is rapidly suppressed in both instances and consequential down-stream signals are engaged.
Negative feedback signals mediate senescence in vivo
Finally, we show that these negative feedback pathways function in vivo in benign human tumors from NF1 patients. Strikingly, in senescent cells within human neurofibromas, AKT and ERK are completely suppressed and FOXO is activated. Accordingly, we hypothesize that these signals underlie the senescence response in these lesions, thereby limiting the growth and progression of these tumors. It has been suggested that senescence might suppress tumor development, if it occurs in a stem or progenitor cell population, resulting in the depletion of this self-renewing pool of cells (Campisi, 2005). Previous studies have not attempted to identify or characterize the senescent population of cells in other benign tumors. In this regard it is notable that the majority of senescent cells in benign human neurofibromas expressed both AP2-α and Krox-20, markers of early Schwann cell progenitors. While the “cancer stem cell” in neurofibromas remains to be identified, these observations indicate that a relatively undifferentiated population of cells can become senescent in these benign human tumors. If these cells represent the cancer stem cell or a critical progenitor population, the senescence response may impede tumor development by eliminating the proliferative capacity of this population of cells. However, the challenge in the future will be to determine how a subset of cells escape this self-limiting response.
Experimental Procedures
Cell culture and infections
Human IMR90 and BJ fibroblasts were sub-cultured 1:2 except prior to infection when they were split 1:3. Wild type and Nf1 −/− MEFs were generated as described (Cichowski et al., 2003). Retroviral and lentiviral infections were performed as described (Johannessen et al., 2005). The following constructs were in the pBabe retroviral vector: H-RasV12, hNF1-GRD-HA, PTEN, p53DD and inducible estrogen receptor-Raf-1 (Raf1:ER). Large T and Large T Δ234–444 were in pMKO.1. Sprouty 2 was in pMSCV. The pLKO.1 lentiviral vector containing the following shRNAs were used:
NF1#1: 5′-CAACAACTTCAATGCAGTCTT-3′
NF1#2: 5′-TTATAAATAGCCTGGAAAAGG-3′
p120RasGAP: 5′-GCTGCCTAACTTATCCATCTT-3′
PTEN: 5′-CGTATACAGGAACAATATTG-3′
For experiments in which control cells were compared shRNA-expressing cells, cells were split to an equal cell density 16 hours prior to harvesting. The following inhibitors were used: 50 μM PD98059 (Calbiochem), 30–50 μM LY294002 (Calbiochem) and 100nM Wortmannin (Sigma-Aldrich)). Wortmannin was re-added to the media every 3 hours during the first 12 hours, the media was changed every 24 hours (and this protocol was repeated daily). Other inhibitors were added daily with fresh media.
Growth curves
IMR90 fibroblasts were infected and selected for 2 days with puromycin (2 μg/ml). 2.0×105 cells were seeded in triplicate in 10-cm plates. Cells were counted and seeded at a density of 2.0×105 every 4 days, for 5 passages. Population doublings (PD) were determined by the formula: PD = Log (Nf/Ni)/Log2, where Nf = the number of cells counted and Ni = the number of cells seeded (2.0×105). Cumulative PD numbers represent the sum of PD’s from all previous passages. For MEFs, 3.0×105 cells were seeded in 6-cm dish, counted every 3 days and reseeded at a density of 3.0×105 (Sage et al., 2000).
SA β-Galactosidase activity
SA β-Gal staining was performed as described (Dimri et al., 1995), and percentages were assessed by counting at least 300 cells. For human neurofibromas, tumor tissue was obtained immediately after surgery, small pieces were fixed for 30 minutes in 3% paraformaldehyde, incubated as described above, then embedded and sectioned.
Immunoblotting
Western analysis and immunoprecipitations were performed as described (Johannessen et al., 2005) using the following antibodies: phospho-Akt, total Akt, phospho-Erk, total ERK, ph-HDM2 (Cell Signaling Technology); Protein Kinase Bα/AKT1, actin, β-Tubulin, RASA3 (Sigma-Aldrich); p120RasGAP (Transduction Laboratories); neurofibromin (Sc-67), p53 (PAb 240 clone), Sprouty2, Sprouty 4, MKP3, RasGRP1, p16 (Santa Cruz Biotechnologies); pRB, p16INK4a (BD Pharmingen); SOS1 (Upstate); p53 (DO7) (Signet).
Histology
Histological sections were cut into 5 μm sections from paraffin-embeded tissue and stained with H + E where indicated. Unmasking was performed with Sodium Citrate buffer pH 6.0. Antibody dilutions and preferred buffers are as follows: 1: 100 NF1 antibody (4° C O/N, sc-67, Santa Cruz), 1:50 (TBST) p16INK4a (Ab-7, MS-1064-P, Neomarkers), 1:100 (PBS) AP-2 alpha (clone 3B5, Developmental Studies Hybridoma Bank, University of Iowa), 1:50 (TBST) Krox-20 (PRB-236P, Covance), 1:50 (TBST) Phospho-p44/42 MAPK (#4376, Cell Signaling Technology), 1:50 (TBST) Phospho-AKT (#3787, Cell Signaling Technology), 1:50 (PBS) FOXO1 (#9462, Cell Signaling Technology). Antibodies were detected using the Vectastain Elite Universal Kit (PK-6200, Vector Laboratories).
Ras activation analysis
Ras-GTP levels were detected using a Ras-activation assay, following manufacturer’s instructions (Upstate Biotechnology).
Luciferase assay
BJ fibroblasts, stably expressing the inducible Raf construct, were seeded in triplicate in 24-well plates, at a density of 105 cells/well and treated with Ethanol or 1 μM of tamoxifen (Calbiochem). Cells were transfected with 0.25 μg of the FKHR (FOXO1) or FKHRL1 (FOXO3) plasmid, 0.5 μg of the Luciferase reporter gene, 0.25 μg of the pRL and 1 μg of carrier DNA. 48 hours later, cells were lysed and one-fifth of the sample was assayed according to the Promega luciferase protocol.
Realtime PCR
Realtime PCR reactions were performed in triplicate using the Assays-on-Demand Taqman® Gene Expression Assays from Applied Biosystems. Probe sets and methods are described in the Supplementary data Figure 5.
Statistical Analysis
All numerical data including error bars represent the mean +/− the standard deviation.
Human Subjects
The Office of Research Protections ruled that this study does not represent human subjects research due exemption 32 CFR 219.101(b)4: the research involved collection or study of existing pathological specimens that are publicly provided without identifiers.
Supplementary Material
Acknowledgments
We thank Lew Cantley, Ben Neel, Anne Brunet, Reuben Shaw, Sheila Thomas, and Jeff Engelman for helpful discussions. We thank the Broad Institute RNAi consortium (TRC) and William Hahn for providing shRNA constructs and William Hahn for the LgT constructs. We thank Martin McMahon for the Raf: ER construct, Jeff Engelman for the PTEN shRNA construct and Nir Hacohen for the Sprouty 2 construct. The microarray analysis was performed by the Biopolymers Facility at Harvard Medical School. This work was supported by grants from the DOD (DAMD17-03-1-0350) and the NCI (R01 CA111754-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Basu TN, Gutmann DH, Fletcher JA, Glover TW, Collins FS, Downward J. Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature. 1992;356:713–715. doi: 10.1038/356713a0. [DOI] [PubMed] [Google Scholar]
- Bollag G, Clapp DW, Shih S, Adler F, Zhang YY, Thompson P, Lange BJ, Freedman MH, McCormick F, Jacks T, Shannon K. Loss of NF1 results in activation of the Ras signaling pathway and leads to aberrant growth in haematopoietic cells. Nat Genet. 1996;12:144–148. doi: 10.1038/ng0296-144. [DOI] [PubMed] [Google Scholar]
- Brachmann SM, Yballe CM, Innocenti M, Deane JA, Fruman DA, Thomas SM, Cantley LC. Role of phosphoinositide 3-kinase regulatory isoforms in development and actin rearrangement. Mol Cell Biol. 2005;25:2593–2606. doi: 10.1128/MCB.25.7.2593-2606.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, Schlegelberger B, Stein H, Dorken B, Jenuwein T, Schmitt CA. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–665. doi: 10.1038/nature03841. [DOI] [PubMed] [Google Scholar]
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichowski K, Jacks T. NF1 tumor suppressor gene function: narrowing the GAP. Cell. 2001;104:593–604. doi: 10.1016/s0092-8674(01)00245-8. [DOI] [PubMed] [Google Scholar]
- Cichowski K, Santiago S, Jardim M, Johnson BW, Jacks T. Dynamic regulation of the Ras pathway via proteolysis of the NF1 tumor suppressor. Genes & Development. 2003;17:449–454. doi: 10.1101/gad.1054703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, Benguria A, Zaballos A, Flores JM, Barbacid M, et al. Tumour biology: senescence in premalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- Corbalan-Garcia S, Yang SS, Degenhardt KR, Bar-Sagi D. Identification of the mitogen-activated protein kinase phosphorylation sites on human Sos1 that regulate interaction with Grb2. Mol Cell Biol. 1996;16:5674–5682. doi: 10.1128/mcb.16.10.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeClue JE, Papageorge AG, Fletcher JA, Diehl SR, Ratner N, Vass WC, Lowy DR. Abnormal regulation of mammalian p21ras contributes to malignant tumor growth in von Recklinghausen (type 1) neurofibromatosis. Cell. 1992;69:265–273. doi: 10.1016/0092-8674(92)90407-4. [DOI] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Waters SB, Holt KH, Pessin JE. SOS phosphorylation and disassociation of the Grb2-SOS complex by the ERK and JNK signaling pathways. J Biol Chem. 1996;271:6328–6332. doi: 10.1074/jbc.271.11.6328. [DOI] [PubMed] [Google Scholar]
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- Ghislain J, Desmarquet-Trin-Dinh C, Jaegle M, Meijer D, Charnay P, Frain M. Characterisation of cis-acting sequences reveals a biphasic, axon-dependent regulation of Krox20 during Schwann cell development. Development. 2002;129:155–166. doi: 10.1242/dev.129.1.155. [DOI] [PubMed] [Google Scholar]
- Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- Gruis NA, van der Velden PA, Sandkuijl LA, Prins DE, Weaver-Feldhaus J, Kamb A, Bergman W, Frants RR. Homozygotes for CDKN2 (p16) germline mutation in Dutch familial melanoma kindreds. Nat Genet. 1995;10:351–353. doi: 10.1038/ng0795-351. [DOI] [PubMed] [Google Scholar]
- Guerra C, Mijimolle N, Dhawahir A, Dubus P, Barradas M, Serrano M, Campuzano V, Barbacid M. Tumor induction by an endogenous K-ras oncogene is highly dependent on cellular context. Cancer Cell. 2003;4:111–120. doi: 10.1016/s1535-6108(03)00191-0. [DOI] [PubMed] [Google Scholar]
- Hahn WC, Dessain SK, Brooks MW, King JE, Elenbaas B, Sabatini DM, DeCaprio JA, Weinberg RA. Enumeration of the simian virus 40 early region elements necessary for human cell transformation. Mol Cell Biol. 2002;22:2111–2123. doi: 10.1128/MCB.22.7.2111-2123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiatt KK, Ingram DA, Zhang Y, Bollag G, Clapp DW. Neurofibromin GAP related domains (GRDs) restore normal growth in Nf1−/− cells. J Biol Chem. 2000 doi: 10.1074/jbc.M009202200. [DOI] [PubMed] [Google Scholar]
- Holtkamp N, Mautner VF, Friedrich RE, Harder A, Hartmann C, Theallier-Janko A, Hoffmann KT, von Deimling A. Differentially expressed genes in neurofibromatosis 1-associated neurofibromas and malignant peripheral nerve sheath tumors. Acta Neuropathol (Berl) 2004;107:159–168. doi: 10.1007/s00401-003-0797-8. [DOI] [PubMed] [Google Scholar]
- Johannessen CM, Reczek EE, James MF, Brems H, Legius E, Cichowski K. The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc Natl Acad Sci U S A. 2005;102:8573–8578. doi: 10.1073/pnas.0503224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HA, Rosenbaum T, Marchionni MA, Ratner N, DeClue JE. Schwann cells from neurofibromin deficient mice exhibit activation of p21ras, inhibition of cell proliferation and morphological changes. Oncogene. 1995;11:325–335. [PubMed] [Google Scholar]
- Kim HJ, Bar-Sagi D. Modulation of signalling by Sprouty: a developing story. Nat Rev Mol Cell Biol. 2004;5:441–450. doi: 10.1038/nrm1400. [DOI] [PubMed] [Google Scholar]
- Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, Majoor DM, Shay JW, Mooi WJ, Peeper DS. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- Ogawara Y, Kishishita S, Obata T, Isazawa Y, Suzuki T, Tanaka K, Masuyama N, Gotoh Y. Akt enhances Mdm2-mediated ubiquitination and degradation of p53. J Biol Chem. 2002;277:21843–21850. doi: 10.1074/jbc.M109745200. [DOI] [PubMed] [Google Scholar]
- Ohtani N, Zebedee Z, Huot TJ, Stinson JA, Sugimoto M, Ohashi Y, Sharrocks AD, Peters G, Hara E. Opposing effects of Ets and Id proteins on p16INK4a expression during cellular senescence. Nature. 2001;409:1067–1070. doi: 10.1038/35059131. [DOI] [PubMed] [Google Scholar]
- Riccardi VM. Neurofibromatosis: phenotype, natural history, and pathogenesis. 2. Baltimore and London: The Johns Hopkins University Press; 1992. [Google Scholar]
- Sage J, Mulligan GJ, Attardi LD, Miller A, Chen S, Williams B, Theodorou E, Jacks T. Targeted disruption of the three Rb-related genes leads to loss of G(1) control and immortalization. Genes Dev. 2000;14:3037–3050. doi: 10.1101/gad.843200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Seshadri T, Campisi J. Repression of c-fos transcription and an altered genetic program in senescent human fibroblasts. Science. 1990;247:205–209. doi: 10.1126/science.2104680. [DOI] [PubMed] [Google Scholar]
- Stewart HJ, Brennan A, Rahman M, Zoidl G, Mitchell PJ, Jessen KR, Mirsky R. Developmental regulation and overexpression of the transcription factor AP-2, a potential regulator of the timing of Schwann cell generation. Eur J Neurosci. 2001;14:363–372. doi: 10.1046/j.0953-816x.2001.01650.x. [DOI] [PubMed] [Google Scholar]
- Sweet-Cordero A, Mukherjee S, Subramanian A, You H, Roix JJ, Ladd-Acosta C, Mesirov J, Golub TR, Jacks T. An oncogenic KRAS2 expression signature identified by cross-species gene-expression analysis. Nat Genet. 2005;37:48–55. doi: 10.1038/ng1490. [DOI] [PubMed] [Google Scholar]
- Tresini M, Mawal-Dewan M, Cristofalo VJ, Sell C. A phosphatidylinositol 3-kinase inhibitor induces a senescent-like growth arrest in human diploid fibroblasts. Cancer Res. 1998;58:1–4. [PubMed] [Google Scholar]
- Tsao H, Goel V, Wu H, Yang G, Haluska FG. Genetic interaction between NRAS and BRAF mutations and PTEN/MMAC1 inactivation in melanoma. J Invest Dermatol. 2004;122:337–341. doi: 10.1046/j.0022-202X.2004.22243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuveson DA, Shaw AT, Willis NA, Silver DP, Jackson EL, Chang S, Mercer KL, Grochow R, Hock H, Crowley D, et al. Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell. 2004;5:375–387. doi: 10.1016/s1535-6108(04)00085-6. [DOI] [PubMed] [Google Scholar]
- Wei S, Sedivy JM. Expression of catalytically active telomerase does not prevent premature senescence caused by overexpression of oncogenic Ha-Ras in normal human fibroblasts. Cancer Res. 1999;59:1539–1543. [PubMed] [Google Scholar]
- Wei W, Hemmer RM, Sedivy JM. Role of p14(ARF) in replicative and induced senescence of human fibroblasts. Mol Cell Biol. 2001;21:6748–6757. doi: 10.1128/MCB.21.20.6748-6757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Woods D, McMahon M, Bishop JM. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 1998;12:2997–3007. doi: 10.1101/gad.12.19.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Ghosh P, Charnay P, Burns DKDK, Parada LP. Neurofibromas in NF1: Schwann Cell Origin and Role of Tumor Environment. Science. 2002;296:920–922. doi: 10.1126/science.1068452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.