Abstract
Eye movements were monitored in 16 women and 20 men during completion of a standard diagram-based test of mental rotation ability to provide measures of cognitive function not requiring conscious, decisional processes. Overall, women and men allocated visual attention during task performance in very similar, systematic ways. However, consistent with previous suggestions that sex differences in attentional processes during completion of the mental rotation task may exist, eye movements in men compared to women indicated greater discrimination and longer processing of correct alternatives during task performance. Other findings suggested that androgens may enhance cognitive processes that are recruited differentially by women and men as a function of the task. Specifically, smaller (i.e., more masculine) digit ratios were associated with men’s shorter fixations on distracters, suggesting that perinatal androgen action may influence brain systems that facilitate the identification of relevant task stimuli. In women, higher circulating testosterone levels appeared to contribute to more general processes engaged during task performance, for example higher levels of visual persistence. It is possible that variability in the relative contribution of such hormone sensitive cognitive processes to accuracy scores as a function of different sample characteristics or assessment methods may partially account for the inconsistent findings of previous research on hormonal factors in mental rotation ability.
Keywords: Sex differences, eye-tracking, spatial ability
In other species, hormonal factors that direct the sexual differentiation of brain substrates in perinatal life and modulate their expression in postnatal life are well-established determinants of sex differences in behavior (Breedlove et al., 1999; Goy and McEwen, 1980). Increasingly, comparative research on human behavior supports the hypothesis that hormonal factors also contribute to sex-linked behavior in children and adults (Cohen-Bendahan et al., 2005; Collaer and Hines, 1995). Yet, evidence for a role of prenatal and postnatal hormones in the development of the large and reliable sex difference in the ability to mentally rotate objects (Linn and Petersen, 1974; Voyer et al., 1995) remains equivocal.
The hypothesis that higher levels of prenatal androgens in men organize brain systems that better support mental rotation ability, for instance, is consistent with recent reports of significant associations between accuracy scores and the ratio of the second to fourth digits in men (Kempel et al., 2005; Manning and Taylor, 2002; McFadden and Shubel, 2003), a putative marker of perinatal androgen action (Brown et al., 2002; Manning et al., 2003; Manning et al., 1998; McIntyre, 2006) and with earlier studies showing enhanced abilities in girls exposed to higher levels of prenatal androgens because of an endocrine disorder (Hampson et al., 1998; Resnick et al., 1986). However, other investigations of sex-linked cognitive behavior find no relationship (Austin et al., 2002; Coolican and Peters, 2003; Poulin et al., 2003) or the opposite association (Putz et al., 2004) between androgen sensitivity (as indicated by digit ratios) and measures of mental rotation ability in women and men. Further, other researchers measuring spatial abilities following atypical prenatal androgen exposure have reported enhanced performance in girls on a targeting task that shows a large male advantage but not on a three-dimensional mental rotation test (Hines et al., 2003). This last finding has suggested that the relative contribution of hormonal and experiential factors to spatial abilities varies across tasks, such that sex-linked factors in postnatal life may be more significant determinants of the sex difference in mental rotation ability.
One possibility is that sex differences in levels of hormones following reproductive maturity contribute to behavioral variability between and within the sexes, presumably by the differential activation of neural systems organized by hormones earlier in prenatal life (Collaer and Hines, 1995). The activational hypothesis is supported by studies showing sex-linked cognitive abilities (including spatial abilities) fluctuate with levels of estradiol and testosterone across the human menstrual cycle (Hampson, 1990a; Hampson, 1990b) and reports of within-sex correlations between hormones and task performance in women and men (Moffat and Hampson, 1996). However, although some menstrual cycle research indicates that mental rotation ability is enhanced during times of low ovarian steroid production (Hausmann et al., 2000), others have found mental rotation ability is unrelated to menstrual cycle phase (Epting and Overman, 1998). Similarly, whereas significant correlations between endogenous testosterone levels and mental rotation ability have been reported in adults (Moffat and Hampson, 1996), more recent research has found that sex-linked spatial abilities, including mental rotation, were unrelated to individual differences in levels of circulating sex steroids and gondotropins in healthy women and men (Halari et al., 2005). Likewise, in research on the behavioral effects of exogenous androgen administration to individuals with androgen deficiencies, marked elevations in circulating levels of hormone were not associated with significant improvements in performance on measures of two-dimensional (Liben et al., 2002) or three-dimensional (Alexander et al., 1998) mental rotation ability.
It is generally assumed that accuracy scores (the primary dependent variable across studies) are measures of the ability to mentally rotate objects. However, accuracy scores are also influenced by decisional processes (Hooven et al., 2004) that may contribute to their apparent sensitivity to factors such as propensity to guess (Voyer and Saunders, 2004), time constraints (Voyer, 1997), socioeconomic status (Levine et al., 2005), and the presence of emotional stimuli (Alexander, 2005). Therefore, a useful strategy in research aiming to better understand the role of hormones in sex differences in mental rotation ability may be to include measures of cognition that are not dependent on conscious, decisional processes during task performance. The potential value of this approach is implied in the previous speculations that attentional processes during mental rotation ability may differ between women and men (Jordan et al., 2002; Peters, 2005) and supported by recent research on mental rotation ability in men suggesting higher levels of circulating testosterone in men may improve performance on mental rotation tasks by enhancing discrimination between different objects (Hooven et al., 2004).
An examination of such processes in the context of hormone-behavior research was accomplished in the present investigation by monitoring eye movements in women and men during completion of a widely used diagram-based test of mental rotation, the redrawn Vandenberg and Kuse Mental Rotations Test (Peters et al., 1995; Vandenberg and Kuse, 1978). The rationale for examining eye movements in research on sex-linked cognitive processes included evidence that eye movement patterns can differentiate between individuals who successfully and unsuccessfully solve a diagram-based problem (Grant and Spivey, 2003), evidence that eye movements are an implicit, unobtrusive measure of performance (Richardson and Spivey, 2000), and evidence that, compared to percentage correct or reaction time measures, eye-movements permit stronger inferences about cognitive function, specifically the allocation of visual attention (Hayhoe, 2004). Indicators of visual attention such as fixation number and fixation duration, for example, are generally thought to reflect visual interest and processing efforts, respectively (Rayner, 1998). We reasoned that because eye-movements are implicit and sensitive to online visual and cognitive processes, eye-movements may reveal sex-linked information processes that, compared to response accuracy, show a stronger association to hormonal factors. We tested this hypothesis by examining whether eye-movements during task performance were associated with salivary levels of sex steroids and digit ratios.
METHOD
Participants
Participants were 16 women and 20 men between 18 and 35 years of age who were enrolled in an introductory psychology course at Texas A&M University. Women and men reported being in good health (i.e., no systemic disease) and none were using hormonal preparations, including hormonal contraceptives. All participants were tested individually in a session lasting approximately 40 minutes. All participants provided signed, informed consent and received partial course credit for their participation in the protocol.
Measures
Cognitive Tasks
Participants first completed a vocabulary test (Ekstrom et al., 1976), a measure of general cognitive ability that typically does not show a sex difference. Mental rotation ability was assessed using the redrawn Vandenberg and Kuse Mental Rotations Test (Peters et al., 1995; Vandenberg and Kuse, 1978) that consists of 24 items, each depicting five line drawings of a three-dimensional block figure. One figure is the target, two figures are the target figure depicted in a different rotation (i.e., correct alternatives) and two figures are distracters. The task instructions (to identify the two rotated versions of the target figure) and time constraints (3 mins per 12 test items separated by a 2 min rest interval) were identical to the paper and pencil version of the task. However, to permit eye-tracking during completion of the test, the 24 items (i.e., each set of five figures) were presented one at a time on a 17 in computer monitor. The size of each resulting array was approximately 2.5 in by 7.5 in, with individual figures sized at approximately 2.5 in by 1.5 in. Participants verbally identified the serial position of the two correct alternatives from left to right (e.g.., “one and four”) before pressing the space bar to advance to the next item. An experimenter recorded verbal responses and later scored the total number of responses identifying both correct items (maximum score: 24), a method that maximizes the sex difference in performance (Voyer et al., 1995).
Eye movement data collection
Eye-movements were monitored using an infra-red eye-tracker with remote optics (Model 504, Applied Science Laboratory) that can measure gaze position with an accuracy of approximately 0.5° of visual angle, a margin of error consistent with the natural function of the human eye. The remote optics system uses corneal and retinal reflections of infra-red light to determine eye gaze with a “bright pupil technology” that minimizes interference from eyeglasses, contacts, and eye-lashes. The camera was situated directly below the computer monitor and participants were seated so that the camera to eye distance was approximately 22 in. A magnetic head tracker (Flock of Birds®, Ascension Technology Corporation) was worn by participants to limit any disruption in eye-tracking as a function of head movement (i.e., no chin rest was required). To obtain valid and reliable eye movement data, 9 gaze positions covering over 80% of the viewing area were first collected from each participant (i.e., a 9-point calibration). Stimulus presentation and data collection (i.e., eye-position) were achieved using GazeTracker™ software (Lankford, 2000). Fixations were defined as a period of at least 100 msec during which point of regard did not change by more than 1-degree visual angle (i.e., a distance on the display of less than 0.5 in).
Hormone Measures
A small amount of saliva (<15 ml) collected by passive drool was obtained from each participant. Prior to the test session, women and men were e-mailed instructions to avoid alcohol and dental work (for 24 hours pre-testing) and to not eat or brush their teeth (for 3 hours pre-testing), restrictions that were later verified by questionnaire. Saliva samples were stored at −80° C, a temperature that compared to −20° C increases the validity of assay results (Granger et al., 2004). Frozen samples were shipped overnight in dry ice to Salimetrics (State College, Pennsylvania), where salivary levels of testosterone (in women and men) and estradiol (in women) were measured in duplicate using enzyme immunoassays.
The ratio of the lengths of the second and fourth digits (2D: 4D) was calculated by obtaining a digital photo scan of the participant’s right hand. Color images of hands were later used to measure the distance in millimeters from the basal crease to the tip of the second and fourth fingers with digital vernier calipers. Two independent judges coded finger-lengths for each hand copy. Measurements averaged across the two judges showed excellent inter-rater reliability (rs >.97), consistent with the findings of previous research using this method of assessment (McIntyre, 2006).
Results
Behavioral and Hormone Measures
There were no outliers (defined as two standard deviations above or below the mean) in the distribution of scores for the self-report behavioral measures and the hormone measures. As shown in Table 1, women and men were similar in age and in their scores on the vocabulary test. As expected, compared to women, men had higher (i.e., more male-typical) salivary levels of free testosterone and higher scores on the test of mental rotation ability. The means for digit ratios in women and men differed in the expected direction (i.e., larger in women) but the trend did not reach statistical significance.
TABLE 1.
Characteristics of women and men, data are means ± SD
| Men (n=20) | Women (n=16) | Cohen’s d | |
|---|---|---|---|
| Age, yrs | 19.10 ±1.41 | 19.18 ± 1.04 | 0.06 |
| Vocabulary, number correct | 21.73 ± 6.96 | 24.62 ± 10.45 | 0.32 |
| Mental Rotations, items completed | 19.50 ± 3.87 | 17.40 ± 5.81* | 0.42 |
| range | 12–24 | 11–24 | |
| mode | 23 | 13 | |
| median | 21 | 18.5 | |
| Mental Rotation, number correct | 13.60 ± 4.60 | 10.73 ± 3.43* | 0.71 |
| 2D:4D Ratio | .958 ±.04 | .970 ±.02 | 0.38 |
| Salivary Testosterone, pg/mL | 208.34 ± 77.10 | 114.83 ± 45.00** | 1.48 |
| Salivary Estradiol, pg/mL | --------- | 13.23 ± 4.67 |
P<.01,
P<.05
Visual Attention
Eye-movement data were based on a successful calibration and a high percentage of successful eye-tracking time (> 95%) in women and men. The five figures associated with each item were defined as areas of interest (AOI). Visual attention in the five AOI was measured in terms of the average number and average duration of visual fixations.
Preliminary analyses
An inspection of the distribution of data values showed no outliers except in the distribution of the average number of fixations on incorrect alternatives (one male above the mean, one female above the mean, and one female below the mean). However, the inclusion or exclusion of these individuals did not significantly impact the direction and significance of the results, and so the analysis of all participant data is reported below.
First, the average amount of time to complete each trial and the percentage of time fixated on stimuli during each trial was calculated for women and men. Men compared to women took less time to complete trials (M = 18.9 ± 5.53 secs vs. M = 20.53 ± 5.88 secs). However, this sex difference in response time was not significant, F(1, 34) =.069, p =.42. Similarly, the percentage of total time fixated on task stimuli during each trial was comparable in men (60.18 ± 16.92 %) and in women (58.65 ± 16.75%), F(1, 34) =.071, p =.79. Next, to ensure that the serial position of visual stimuli did not have differential effects on the patterns of eye-movements in women compared to men, separate ANOVAs with sex as a grouping factor and serial position from left to right (1 to 5) as a repeated measure were performed on visual attention measures. The analysis of fixation number showed a main effect of serial position, F(4, 31) = 39.54, p <.001, but no significant main effect of sex, F(1, 34) = 0.55, p =.46, and no significant sex by serial position interaction effect, F(4, 31) = 2.90, p =.08. The analysis of fixation duration also showed the serial position effect, F(4, 31) = 4.56, p <.001, but no main effect of sex, F(1, 34) = 0.01, p =.84 and no sex by serial position interaction effect, F(4, 31) = 0.99, p =.42. The main effects of serial position on visual attention measures reflected the finding that a larger number of fixations and a longer duration of fixations on the first figure (i.e., the target) occurred compared to the four choice items (all ps <.01). Notably, measures of visual attention directed to the target figures were comparable in women and men (fixation number: M=10.13 ± 4.61 for men vs. M = 9.16 ± 3.64 for women, t(34) =.68, p =.50; fixation duration: M=0.39 secs ± 0.10 secs for men vs. M = 0.43 secs ± 0.13 secs for women, t(34) =−.92, p =.36). Post hoc analyses using Tukey’s tests for multiple comparisons also showed that in both sexes a smaller number and shorter duration of visual fixations on the figure in the last (i.e., extreme right) location occurred (p <.01). These effects of serial position on visual attention measures are consistent with a decision making process that involves a consideration of each item according to its serial position and then is terminated after identifying two correct or two incorrect items. Importantly, there was no evidence that this pattern of eye-movements differed between women and men.
Sex Differences in Visual Attention
As noted above, no significant sex differences were observed in response times, in the percentage of total time fixated on stimuli during each trial, and in measures of visual attention directed to task stimuli defined according to serial position. Therefore, sex differences in visual attention to correct alternatives and distracters during performance of the test of mental rotation ability were examined by averaging measures of fixation and duration for each figure type using ANOVA for repeated measures with sex (male vs. females) as a grouping factor and figure type (correct alternatives vs. distracters) as the within-subject factor. However, because men showed marginally shorter response times and completed significantly more items than did women, a second analysis tested specifically for sex differences in the percentage of total fixations and percentage of total fixation time for the trial in each AOI (correct and incorrect alternatives).
Fixation Number
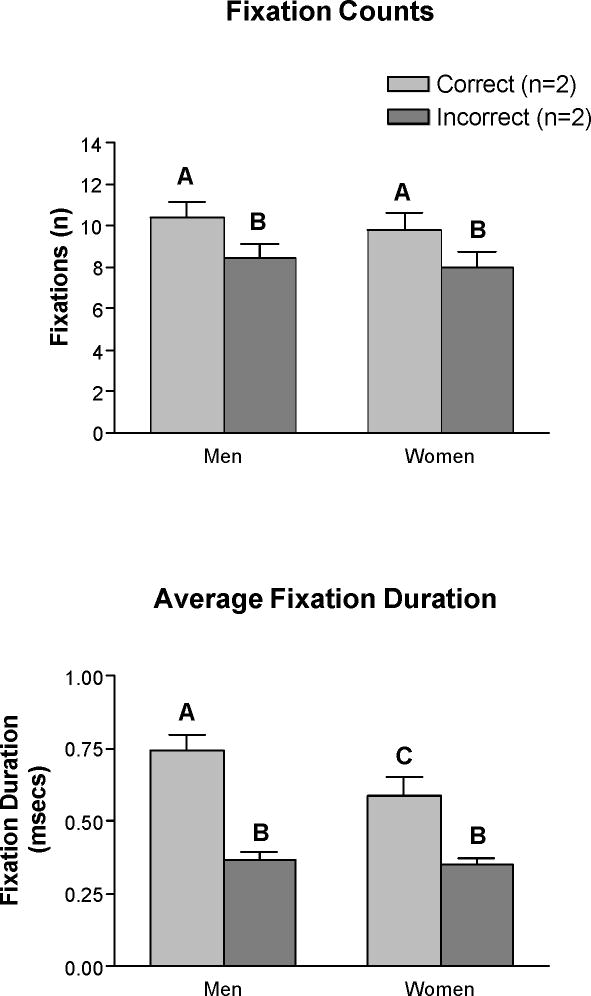
The analysis of all completed trials using sex (women vs. men) as a grouping factor and the number of items completed as a covariate showed a main effect of figure type, F(1, 31) = 4.65, p <.05, but no interaction effect (all Fs < 1.0) and no main effect of sex, F(1, 31) = 0.36, p =.55. The main effect of figure type reflects the finding that the average number of fixations on correct alternatives was generally greater than the number of fixations on distracters (Figure 1). The between-sex comparison of the relative number of fixations on figure type showed the percentage of total fixations on correct alternatives was similar in women and men (M = 18.60% ± 2.95% for women vs. M = 17.64% ± 3.31% for men), t (34) = 0.89, p =.38. However, relative to women, men directed a smaller percentage of their total fixations to the incorrect alternative (M = 14.30% ± 2.95% vs. M = 15.99% ± 2.59%, t (34) = 2.07, p <.05).
Figure 1.
Mean (±SEM) number and duration of fixations on correct alternatives and distracters by sex across all completed items. Means with the same letter name are not significantly different.
Fixation Duration
The analysis of fixation duration across all completed trials showed a main effect of figure type, F(1, 33) = 26.24, p <.001, and a sex by figure type interaction, F(1, 33) = 4.07, p =.05. The main effect of sex on performance was not significant, F(1, 33) = 2.67, p =.11. The main effect of figure type resulted because fixation durations were longer on correct alternatives than on distracters in both sexes. However, posthoc analysis showed that the interaction between sex and figure type occurred because men compared to women showed longer fixations on correct alternatives, p <.05 (Figure 1). The between-sex comparison of the relative duration of time in each AOI showed similar results. Whereas the percentage of time in the zone defined as incorrect alternatives did not differ between women and men (M = 11.66% ± 2.15% for men vs. M = 11.48% ± 2.42% for women), the percentage of time in the zone defined as correct alternatives was greater in men (M = 14.68% ± 2.58%) than in women (M = 12.89% ± 2.57%), t (34) = 2.06, p <.05.
Within-sex correlational analysis: eye-movements and hormonal measures
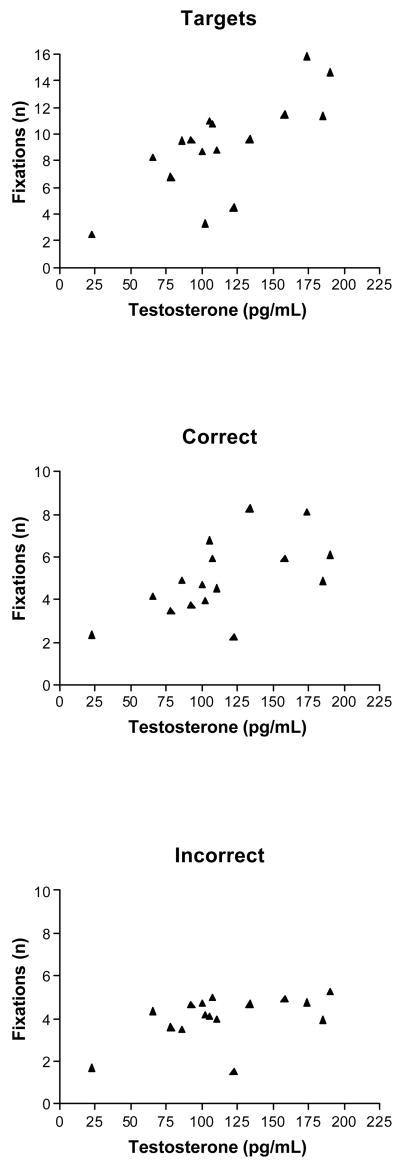
As some previous researchers have demonstrated curvilinear associations between circulating testosterone levels and spatial abilities (Moffat and Hampson, 1996; but see Alexander et al., 1998), we visually inspected the data for nonlinear trends and also calculated the association between behavior and the quadratic transformation of the deviation from the mean testosterone level. Although there were no significant nonlinear hormone-behavior associations, Table 2 summarizes the significant linear associations (as measured by Pearson product moment correlations) among response accuracy, average measures of visual attention, and measures of digit ratio and postnatal testosterone levels. In men only, higher levels of response accuracy were significantly correlated with less visual attention on task stimuli. In particular, the association between fixation number on targets and accuracy scores in men, r(20) =−.58, p <.05, was significantly different from the association between these variables in women, r(16) = 0.06, Fisher’s z-test = −1.96, p <.05. In men, smaller (i.e., more masculine) digit ratios were associated with shorter fixations on distracters (p <.05), but this association was not significantly different than that observed in women. In contrast, as illustrated in Figure 2, salivary testosterone levels in women were positively associated with both the number of fixations on targets, r(16) = 0.74, p <.01, and the number of fixations on correct alternatives, r(16) =.59, p <.05, a pattern of results that was significantly different from the correlations found between testosterone levels in men and the number of fixations on targets, r(20) = −.02, and correct alternatives, r(20) = −.11, Fisher’s z-test = −2.63, −2.03, p <.01 and.05, respectively. No significant correlations between estradiol levels in women and behavior were found. Correlations between sex steroid levels and measures of the relative amount of attention directed to correct alternatives and distracters were also small and nonsignificant.
TABLE 2.
Within sex correlations between sex-linked measures and average measures of visual attention during performance of the mental rotations test
| MRT Score | Digit Ratio | Testosterone | Estradiol | ||
|---|---|---|---|---|---|
| Men (n=20) | |||||
| MRT | −0.06 | −0.20 | -- | ||
| Testosterone | 0.23 | ||||
| Fixations, n | Targets | −0.58* | 0.15 | −0.02 | -- |
| Correct alternatives | −0.33 | 0.23 | −0.11 | -- | |
| Distracters | −0.51* | 0.08 | 0.11 | -- | |
| Fixations, duration | Targets | −0.49* | 0.43 | 0.17 | -- |
| Correct alternatives | −0.55* | 0.19 | −0.04 | -- | |
| Distracters | −0.38 | 0.47* | 0.21 | -- | |
| Women (n=16) | |||||
| MRT | 0.34 | 0.14 | 0.37 | ||
| Testosterone | 0.01 | ||||
| Fixations, n | Targets | 0.06 | 0.05 | 0.74* | 0.09 |
| Correct alternatives | −0.26 | 0.25 | 0.59* | 0.21 | |
| Distracters | −0.23 | 0.25 | 0.49 | −0.06 | |
| Fixations, duration | Targets | 0.07 | −0.14 | 0.15 | −0.03 |
| Correct alternatives | −0.34 | −0.01 | 0.52* | −0.08 | |
| Distracters | −0.24 | 0.23 | 0.05 | −0.32 | |
P<.05
Figure 2.
Scatterplots illustrating the positive linear associations between measures of testosterone in women and visual attention directed to targets (top) and correct alternatives (center).
To assess further the apparent specificity of the observed linear associations between hormone measures and visual attention in women and men, correlations between hormone measures and the serial position of alternatives from left to right were also calculated. In men, there were no significant associations between digit ratios and fixation number or the average duration of fixations on the four alternatives (all rs <.30). However, in women significant corrections were observed between salivary testosterone and the number of fixations on alternatives early in serial position (Alternative 1: r(16) =.69, p <.01; Alternative 2: r(16) =.51, p <.05; Alternative 3: r(16) =.48, p =.06; Alternative 4: r(16) =.23, p =.39). Testosterone levels in women and the average fixation duration on the four alternatives were unrelated (all rs <.34).
DISCUSSION
Eye movements in women and men were monitored during completion of a diagram-based test of mental rotation ability that typically shows a large sex difference in response accuracy. Consistent with our hypothesis, global measures of visual attention directed to the test stimuli during task performance in this research were sensitive to hormonal factors even though accuracy scores were not. These findings suggest that the contribution of hormonal factors to the underlying cognitive processes recruited by women and men during the mental rotation test may be larger than their contribution to accuracy scores, perhaps because accuracy scores reflect the sum of a variety attentional and decisional processes (Hooven et al., 2004; Voyer, 1997). If so, then variability in the relative contribution of these hormone sensitive processes to accuracy scores (for example, as a consequence of sample characteristics or study design) may partially account for the inconsistent findings of previous research on hormonal factors in mental rotation ability.
It is noteworthy that women and men in this research allocated visual attention during task performance in very similar, systematic ways. Specifically, sex differences were not found in the percentage of time fixated on task stimuli during each trial or on the number or duration of visual fixations on the five figures when considered only in terms of their serial position. Both sexes directed more visual attention to the target figure relative to the four comparison figures. Further, in both women and men, comparison figures located last in serial position received a smaller amount of visual attention relative to those figures located earlier in serial position, a pattern of response that is consistent with a decision making process that terminates after identifying two correct or incorrect exemplars of the target figure. Perhaps of greater theoretical interest than these results for serial position and attention, women and men also allocated more visual attention to the two figures that were correct alternatives compared to the two other figures that were distracters. Fixation number and fixation duration, the measures derived from the analysis of eye movements used in this research, are generally thought to reflect visual interest and processing efforts, respectively (Rayner, 1998). For example, other research of visual attention has shown that chess experts fixated proportionally more on relevant pieces than non-expert players (Charness et al., 2001) and successful and unsuccessful problem solvers can be differentiated in terms of the allocation of visual attention to relevant features of a diagram-based problem (Grant and Spivey, 2003). Therefore, the present findings showing that both sexes directed greater visual attention or processing efforts to the two comparison figures associated with the successful completion of the mental rotations test are consistent with a fundamental assumption underlying this research, namely that eye movements are sensitive to cognitive processes engaged during complex cognitive tasks.
A sex difference stands against the background of many similar, essential elements of a behavior (i.e., the behavior is identifiable in women and men, but may differ in its frequency, strength, or efficiency). It is interesting, therefore, that although eye movement measures were generally comparable in women and men, other findings from this research support the previous speculations that some attentional processes during completion of the mental rotation task may differ between women and men (Jordan et al., 2002; Peters, 2005). One finding was that the proportion of fixations on correct alternatives was smaller in men than in women. A second finding was that fixations on correct alternatives were generally longer in men compared to women, although both sexes looked longer at correct alternatives. An interpretation of these results in the context of eye movements and cognitive processes is that men show a greater discrimination and a generally longer or deeper processing of correct alternatives during completion of the mental rotation task.
Men (with generally better accuracy scores than women) showed longer fixations on correct alternatives than did women. However, shorter fixations on correct alternatives were associated with better accuracy in both sexes (although the trend in women did not reach statistical significance). To our knowledge, this is the first report of eye movements in women and men during performance of a mental rotation task and so any interpretation of these apparently inconsistent results must include a measure of speculation. To visualize the findings, imagine the correlation between response accuracy (x-axis) and fixation duration (y-axis) in women and men as one negatively sloped line positioned above another. A reasonable interpretation of the negative slopes is that a greater efficiency in extracting information is advantageous to task performance in men and to some degree in women. A more speculative interpretation of the higher position of the line for men on the y-axis (i.e., the longer fixations in men) is that women and men differ in the type of information that they are extracting from the visual stimulus.
Sex differences in the efficacy or the type of cognitive processes engaged during the mental rotation task are implicated in research showing sex differences in the areas of brain activation (Butler et al., 2006; Jordan et al., 2002) or in the intensity of brain activation (Halari et al., 2006) during completion of the mental rotation task. One suggestion is that sex differences in spatial processing may occur because women show a processing bias for more relative spatial information (i.e., the relationship among parts of a whole), whereas men show a processing bias for global or absolute spatial information (Alexander, 2003; Alexander et al., 2002; Collaer and Hill, 2006; Rybash and Hoyer, 1992). Interestingly, research measuring eye movements has shown that global analysis (i.e., the hypothesized male-typical strategy) compared to attention to object parts (i.e., the hypothesized female-typical strategy) activates a more focused attention (Weber et al., 2000), consistent with the relatively longer fixation durations of men in this research. Although this proposal is tentative, by examining the numbers of components of the block-like stimuli that are fixated and their duration (i.e., a part or whole analyses) when making comparison among alternatives, it may be possible in future research to determine whether such sex-linked strategies are applied during the test of mental rotation ability and whether they can account for the present results.
Sex differences in behaviors are thought to be determined in part by hormonal factors. Therefore, it may be important in understanding the observed sex differences in eye movements that the correlational analysis suggests that cognitive processes activated during the test of mental rotation ability are also hormone sensitive. In men, smaller (i.e., more male-typical) digit ratios were associated with less visual attention on distracters. In women, higher levels of salivary testosterone were associated with a greater number of fixations on multiple areas of interest. Digit ratios appear to be a useful indicator of perinatal androgen action (for review, see McIntyre, 2006). If so, then our data suggest that greater sensitivity to androgens may enhance brain systems that are recruited during the identification or rejection of nonrelevant task stimuli, and that the efficacy of these systems may be important for successful male-typical strategies. The more generalized pattern of correlations observed in women suggests circulating androgens may increase broad factors, such as motivation, visual or attentional persistence, consistent with recent functional neuroimaging research indicating that women compared to men use “top-down” effortful processing during performance of the mental rotation task (Butler et al., 2006) and may recruit more brain resources before obtaining performance levels comparable to men (Halari et al., 2006). Interestingly, women’s eye movements were unrelated to accuracy scores, suggesting that processes that may be sensitive to salivary testosterone levels in women are not reliable predictors of correct solutions to the task problems. However, other more critical factors being constant, hormonal effects on general processes such as visual persistence or attention may contribute to the within-subject improvement in mental rotation ability reported following testosterone administration to young, healthy women (Aleman et al., 2004) and to women with low levels of sex steroids because of anorexia nervosa (Miller et al., 2005). Future research examining task related eye movements prior to and following hormone administration may be informative in this regard.
Finally, we argue that the results of this research support a role for hormonal influences on cognitive processing during the mental rotation task. However, our interpretations are subject to some caveats. The relatively small number of participants likely limited our power to detect significant associations between estradiol and behavior and between sex steroids and accuracy scores. Our procedures also required a verbal response from participants which may have increased anxiety and influenced our behavioral results for women and men. Further, findings that increased experience with blocks enhances young children’s subsequent recognition and reproduction of geometric shapes (Sprafkin et al., 1983) and reports of small but significant associations between experiences with blocks and other male-typical toys and adult spatial ability, including mental rotation ability (Baenninger and Newcombe, 1989; Voyer et al., 2000) suggest practice or familiarity may also influence abilities to identify task relevant features. Future research using larger numbers of participants and measuring how visual attention may change across trials or how prior experience with blocks or geometric shapes may influence eye movement measures may help better identify sex-linked factors in postnatal development that influence sex differences in mental rotation ability.
In sum, this research using very global measures of visual attention provides new evidence supporting the hypothesis that components of processing during the mental rotation task are sensitive to both sex and hormonal factors. By incorporating a finer analysis of eye movements in future research on sex-linked cognitive abilities, such as that used in research on cognitive and perceptual processes associated with reading (Rayner, 1998), it may be possible to identify more precisely how biological and social factors contribute to human sex differences in tests of mental rotation ability.
Acknowledgments
This research was supported in part by National Institute of Mental Health grant MH071414 (GMA). The authors thank Mark G. Packard for helpful comments on an earlier version of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aleman A, Bronk E, Kessels RPC, Koppeschaar HPF, Van Honk J. A single administration of testosterone improves visuospatial ability in young women. Psychoneuroendocrinology. 2004;29:612–617. doi: 10.1016/S0306-4530(03)00089-1. [DOI] [PubMed] [Google Scholar]
- Alexander GM. An evolutionary perspective of sex-typed toy preferences: Pink, blue and the brain. Arch Sex Behav. 2003;32:7–14. doi: 10.1023/a:1021833110722. [DOI] [PubMed] [Google Scholar]
- Alexander GM. Memory for face locations: emotional processing alters spatial abilities. Evolution and Human Behavior. 2005;26:352–362. [Google Scholar]
- Alexander GM, Packard MG, Peterson BS. Sex and spatial position effects on object location memory following intentional learning of object identities. Neuropsychologia. 2002;40:1516–1522. doi: 10.1016/s0028-3932(01)00215-9. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Swerdloff RS, Wang C, Davidson T, McDonald V, Steiner B, Hines M. Androgen-behavior correlations in hypogonadal men and eugonadal men. II Cognitive abilities. Horm Behav. 1998;33:85–94. doi: 10.1006/hbeh.1998.1439. [DOI] [PubMed] [Google Scholar]
- Austin EJ, Manning JT, McInroy K, Mathews E. A preliminary investigation of the associations between personality, cognitive ability and digit ratio. Personality and Individual Differences. 2002;33:1115–1124. [Google Scholar]
- Baenninger M, Newcombe N. The role of experience in spatial test performance: a meta-analysis. Sex Roles. 1989;20:327–344. [Google Scholar]
- Breedlove SM, Cooke BM, Jordan CL. The orthodox view of brain sexual differentiation. Brain Behav Evol. 1999;54:8–14. doi: 10.1159/000006607. [DOI] [PubMed] [Google Scholar]
- Brown WM, Hines M, Fane BA, Breedlove SM. Masculinized finger length patterns in human males and females with congenital adrenal hyperplasia. Horm Behav. 2002;42:380–386. doi: 10.1006/hbeh.2002.1830. [DOI] [PubMed] [Google Scholar]
- Butler T, Imperato-McGinley J, Pan H, Voyer D, Cordero J, Zhu Y, Stern E, Silbersweig D. Sex differences in mental rotation: Top-down versus bottom-up processing. Neuroimage. 2006 doi: 10.1016/j.neuroimage.2006.03.030. in press. [DOI] [PubMed] [Google Scholar]
- Charness N, Reingold EM, Pomplun M, Stampe DM. The perceptual aspect of skilled performance in chess: Evidence from eye movements. Mem Cognit. 2001;29:1146–1152. doi: 10.3758/bf03206384. [DOI] [PubMed] [Google Scholar]
- Cohen-Bendahan CCC, van de Beek C, Berenbaum SA. Prenatal sex hormone effects on child and adult sex-typed behavior: methods and findings. Neurosci Biobehav Rev. 2005;29:353–384. doi: 10.1016/j.neubiorev.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Collaer ML, Hill EM. Large sex difference in adolescents on a timed line judgment task: attentional contributors and task relationship to mathematics. Perception. 2006;35:561–572. doi: 10.1068/p5003. [DOI] [PubMed] [Google Scholar]
- Collaer ML, Hines M. Human behavioral sex differences: A role for gonadal hormones during early development? Psychol Bull. 1995;118:55–107. doi: 10.1037/0033-2909.118.1.55. [DOI] [PubMed] [Google Scholar]
- Coolican J, Peters M. Sexual dimorphism in the 2D/4D ratio and its relation to mental rotation performance. Evolution and Human Behavior. 2003;24:179–183. [Google Scholar]
- Ekstrom RB, French JW, Harman HH. Kit of Factor Referenced Cognitive Tests. Educational Testing Service; Princeton, NJ: 1976. [Google Scholar]
- Epting LK, Overman WH. Sex-sensitive tasks in men and women: a search of performance fluctuations across the menstrual cycle. Behavioral Neuroscience. 1998;112:1304–1317. doi: 10.1037//0735-7044.112.6.1304. [DOI] [PubMed] [Google Scholar]
- Goy RW, McEwen BS. Sexual Differentiation of the Brain. MIT Press; Cambridge, Massachusetts: 1980. [Google Scholar]
- Granger DA, Shirtcliff EA, Booth A, Kivlighan KT, Schwartz EB. The “trouble” with salivary testosterone. Psychoneuroendocrinology. 2004;29:1229–1240. doi: 10.1016/j.psyneuen.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Grant ER, Spivey MJ. Eye movements and problem solving: guiding attention guides thought. Psychological Science. 2003;14:462–466. doi: 10.1111/1467-9280.02454. [DOI] [PubMed] [Google Scholar]
- Halari R, Hines M, Kumari V, Mehrotra R, Wheeler M, Ng V, Sharma T. Sex differences and individual differences in cognitive performance and their relationship to endogenous gonadal hormones and gonadotropins. Behavioral Neuroscience. 2005;119:104–117. doi: 10.1037/0735-7044.119.1.104. [DOI] [PubMed] [Google Scholar]
- Halari R, Sharma T, Hines M, Andrew D, Simmons A, Kumari V. Comparable fMRI activity with differential behavioural performance on mental rotation and overt verbal fluency in healthy men and women. Experimental Brain Research. 2006;169:1–14. doi: 10.1007/s00221-005-0118-7. [DOI] [PubMed] [Google Scholar]
- Hampson E. Estrogen-related variations in human spatial and articulatory-motor skills. Psychoneuroendocrinology. 1990a;15:97–111. doi: 10.1016/0306-4530(90)90018-5. [DOI] [PubMed] [Google Scholar]
- Hampson E. Variations in sex-related cognitive abilities across the menstrual cycle. Brain & Cognition. 1990b;14:26–43. doi: 10.1016/0278-2626(90)90058-v. [DOI] [PubMed] [Google Scholar]
- Hampson E, Rovet JF, Altmann D. Spatial reasoning in children with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Developmental Neuropsychology. 1998;14:299–320. [Google Scholar]
- Hausmann M, Slabbekoorn D, Van Goozen SHM, Cohen-Kettenis PT, Gunturkun O. Sex hormones affect spatial abilities during the menstrual cycle. Behavioral Neuroscience. 2000;114:1245–1250. doi: 10.1037//0735-7044.114.6.1245. [DOI] [PubMed] [Google Scholar]
- Hayhoe MM. Advances in relating eye movements and cognition. Infancy. 2004;6:267–274. doi: 10.1207/s15327078in0602_7. [DOI] [PubMed] [Google Scholar]
- Hines M, Fane BA, Pasterski VL, Mathews GA, Conway GS, Brooke C. Spatial abilities following prenatal androgen abnormality: targeting and mental rotations performance in individuals with Congenital Adrenal Hyperplasia. Psychoneuroendocrinology. 2003;28:1010–1026. doi: 10.1016/s0306-4530(02)00121-x. [DOI] [PubMed] [Google Scholar]
- Hooven CK, Chabris CF, Ellison PT, Kosslyn SM. The relationship of male testosterone to components of mental rotation. Neuropsychologia. 2004;42:782–790. doi: 10.1016/j.neuropsychologia.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Jordan K, Wustenberg T, Heinze HJ, Peters M, Jancke L. Women and men exhibit different cortical activation patterns during mental rotation tasks. Neuropsychologia. 2002;40:2397–2408. doi: 10.1016/s0028-3932(02)00076-3. [DOI] [PubMed] [Google Scholar]
- Kempel P, Gohlke B, Klempau J, Zinsberger P, Reuter M, Hennig J. Second-to-fourth digit length, testosterone and spatial ability. Intelligence. 2005;33:215–230. [Google Scholar]
- Lankford C. GazeTracker: Software designed to facilitate eye movement analysis. ACM Press; New York: 2000. [Google Scholar]
- Levine SC, Vasilyeva M, Lourenco SF, Newcombe NS, Huttenlocher J. Socioeconomic status modifies the sex difference in spatial skill. Psychological Science. 2005;16:841–845. doi: 10.1111/j.1467-9280.2005.01623.x. [DOI] [PubMed] [Google Scholar]
- Liben LS, Susman EJ, Finkelstein JW, Chinchilli VM, Kunselman S, Schwab J, et al. The effects of sex steroids on spatial performance: a review and an experimental clinical investigation. Developmental Psychology. 2002;35:236–253. [PubMed] [Google Scholar]
- Linn MC, Petersen AC. Emergence and characterization of sex differences in spatial ability: A meta-analysis. Child Development. 1974;56:1479–1498. [PubMed] [Google Scholar]
- Manning JT, Bundred PE, Newton DJ, Flanagan BF. The second to fourth digit ratio and variation in the androgen receptor gene. Evolution and Human Behavior. 2003;24:399–405. [Google Scholar]
- Manning JT, Scott D, Wilson J, Lewis-Jones DI. The ratio of 2nd to 4th digit length: a predictor of sperm numbers and concentrations of testosterone, luteinizing hormone and oestrogen. Hum Reprod. 1998;13:3000–3004. doi: 10.1093/humrep/13.11.3000. [DOI] [PubMed] [Google Scholar]
- Manning JT, Taylor R. Second to fourth digit ratio and male ability in sport: implications for sexual selection in humans. Evolution and Human Behavior. 2002;21:61–69. doi: 10.1016/s1090-5138(00)00063-5. [DOI] [PubMed] [Google Scholar]
- McFadden D, Shubel E. The relationships between otoacoustic emissions and relative lengths of fingers and toes in humans. Horm Behav. 2003;43:421–429. doi: 10.1016/s0018-506x(03)00014-x. [DOI] [PubMed] [Google Scholar]
- McIntyre MH. The use of digit ratios as markers for perinatal androgen action. Reproductive Biology and Endocrinology. 2006;4:10–18. doi: 10.1186/1477-7827-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KK, Grieco KA, Klibanski A. Testosterone administration in women with anorexia. The Journal of Clinical Endocrinology and Metabolism. 2005;90:1428–1433. doi: 10.1210/jc.2004-1181. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Hampson E. A curvilinear relationship between testosterone and spatial cognition in humans: Possible influence of hand preference. Psychoneuroendocrinology. 1996;21:323–337. doi: 10.1016/0306-4530(95)00051-8. [DOI] [PubMed] [Google Scholar]
- Peters M. Sex differences and the factor of time in solving Vandenberg and Kuse mental rotation problems. Brain Cogn. 2005;57:176–184. doi: 10.1016/j.bandc.2004.08.052. [DOI] [PubMed] [Google Scholar]
- Peters M, Laing B, Latham D, Jackson M, Zaiyouna R, Richardson C. A redrawn Vandenberg & Kuse mental rotations test: different versions and factors that affect performance. Brain & Cognition. 1995;28:39–58. doi: 10.1006/brcg.1995.1032. [DOI] [PubMed] [Google Scholar]
- Poulin M, O’Connell RL, Freeman LM. Picture recall skills correlate with 2D:4D ratio in women but not in men. Evolution and Human Behavior. 2003;25:174–181. [Google Scholar]
- Putz DA, Gaulin SJC, Sporter RJ, McBurney DH. Sex hormones and finger length: what does 2D:4D indicate? Evolution and Human Behavior. 2004;25:182–199. [Google Scholar]
- Rayner K. Eye-movements in reading and information processing: 20 years of research. Psychological Bulletin. 1998;124:372–422. doi: 10.1037/0033-2909.124.3.372. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Berenbaum SA, Gottesman II, Bouchard TJ. Early hormonal influences on cognitive functioning in congenital adrenal hyperplasia. Developmental Psychology. 1986;22:191–198. [Google Scholar]
- Richardson D, Spivey M. Representation, space, and Hollywood Squares: Looking at things that aren’t there anymore. Cognition. 2000;76:269–295. doi: 10.1016/s0010-0277(00)00084-6. [DOI] [PubMed] [Google Scholar]
- Rybash JM, Hoyer WJ. Hemispheric specialization for categorical and coordinate spatial representations: a reappraisal. Mem Cognit. 1992;20:271–276. doi: 10.3758/bf03199664. [DOI] [PubMed] [Google Scholar]
- Sprafkin C, Serbin LA, Denier C, Connor JM. Sex-differentiated play: Cognitive consequences and early interventions. In: Liss MB, editor. Social and cognitive skills: Sex roles and children’s play. Academic Press; New York: 1983. pp. 167–192. [Google Scholar]
- Vandenberg SG, Kuse AR. Mental rotation, a group test of three-dimensional spatial visualisation. Perceptual & Motor Skills. 1978;47:599–604. doi: 10.2466/pms.1978.47.2.599. [DOI] [PubMed] [Google Scholar]
- Voyer D. Scoring procedure, performance factors, and magnitude of sex differences in spatial performance. Am J Psychol. 1997;110:259–276. [PubMed] [Google Scholar]
- Voyer D, Nolan C, Voyer S. The relation between experience and spatial performance in men and women. Sex Roles. 2000;43:891–915. [Google Scholar]
- Voyer D, Saunders KA. Gender differences on the mental rotations test: a factor analysis. Acta Psychol (Amst) 2004;117:79–94. doi: 10.1016/j.actpsy.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Voyer D, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: a metal-analysis and consideration of critical variables. Psychological Bulletin. 1995;117:250–270. doi: 10.1037/0033-2909.117.2.250. [DOI] [PubMed] [Google Scholar]
- Weber B, Schwarz U, Kneifel S, Treyer V, Buck A. Hierarchical visual processing is dependent on the oculomotor system. Neuroreport. 2000;11:241–247. doi: 10.1097/00001756-200002070-00004. [DOI] [PubMed] [Google Scholar]