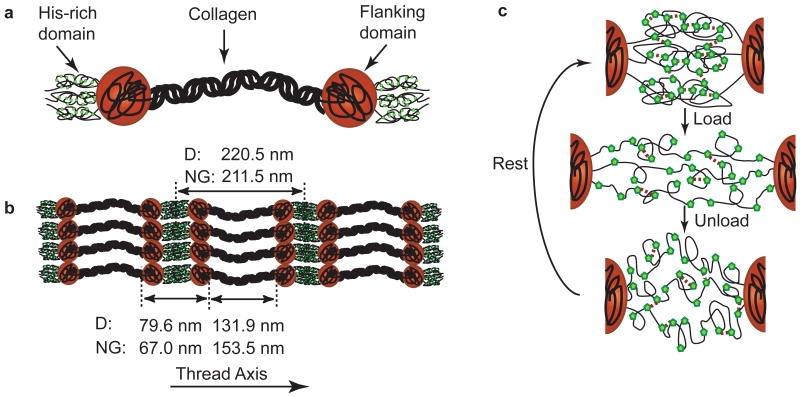
Figure 3. Schematic of preCol morphology, assembly, and behavior under load.
a) PreCols are block co-polymer-like proteins with clearly defined modules including a central bent-core collagen domain, adjacent flanking domains, and terminal histidine-rich domains. The rigid rod-like collagen domains have a well-defined triple helical structure and make up about half of the protein by amino acid quantity. The flanking domains of preCol-D are reminiscent of dragline-silk and are likely more tightly folded than the glycine-rich domains of preCol-NG. His-rich domains at the N- and C-termini of preCols form coordination complexes with transition metals, which are believed to stabilize the domains. PreCol-D and -NG domain lengths were calculated based on sequence data and AFM measurements. b) PreCols are observed to assemble into highly ordered smectic arrays in series with C2 symmetry (Hassenkam et al., 2004). When tensile load is applied to threads, it is transferred along the long axial filaments of preCol. (c) illustrates a molecular level schematic model of His-dependent healing in threads. Green pentagons represent “sticky” His residues interspersed in the amorphous chains of the His-rich domains between two adjacent preCol triple helices. For simplicity of representation in the schematic, a cross-link is formed between two His residues; however, in reality a coordination cross-link would require 3-4 histidines as ligands. In the virgin state prior to applied yield, the His-rich domains are folded in such a way as to supply the largest amount of resistance under tensile load (highest stiffness). When extended past yield, many of the cross-links are ruptured, allowing the folded chains to extend and reveal hidden contour length. The sacrificial breaking of these bonds prevents catastrophic failure of the thread and provides the characteristic hysteretic behavior of the distal portion of byssal threads. When the load is removed, the His-rich domain returns to its initial length, but is not immediately refolded to the “ideal” state. Through stochastic vibrations of the His-rich chains, the His-residues eventually form stable cross-links and over time will recover to a stiff conformation.