Figure 1.
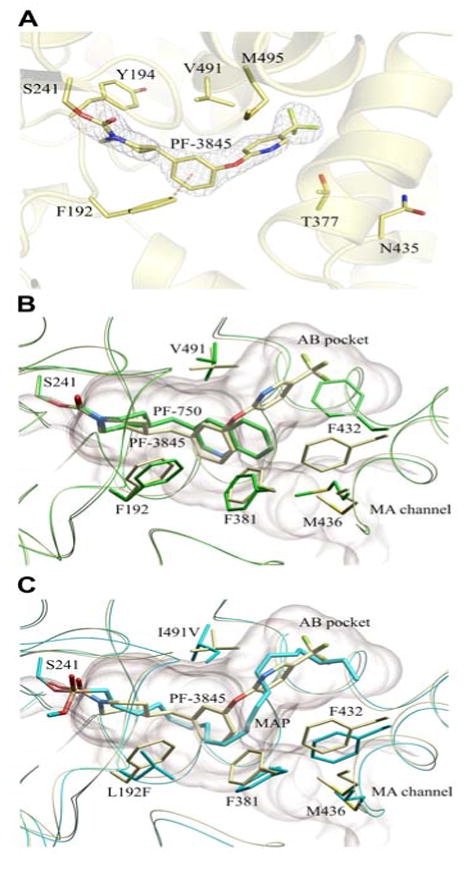
Crystal structure of a PF-3845-h/rFAAH complex. (A) Active site image of PF-3845-h/rFAAH complex, showing the S241-carbamylated adduct and six residues that have been mutated in h/rFAAH. (B) Overlap of the crystal structures of the PF-3845 (gray) and PF-750 (green) complexes with h/rFAAH, showing the different modes of binding that lead to distinct conformations for the F432 residue that toggles between the membrane access (MA) channel (F432 in gray) and AB pocket (F432 in green). (C) Overlap of crystal structures of PF-3845-h/rFAAH and MAP-rFAAH complexes, showing similar binding modes for PF-3845 (gray) and MAP (blue).
