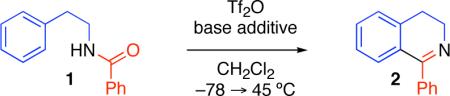
Table 1.
Selection of base additive.a
 | |||
|---|---|---|---|
| entry | base additive | equiv | isolated yield (%) |
| 1 | Et3N | 1.2 | 18 |
| 2 | pyridine | 1.2 | 65 |
| 3 | ethyl nicotinate | 1.2 | 51 |
| 4 | 2-bromopyridine | 1.2 | 74 |
| 5 | 2-fluoropyridine | 1.2 | 90 |
| 6 | 3-chloropyridine | 1.2 | 79 |
| 7 | 2-chloropyridine | 1.2 | 95 |
| 8 | 2-chloropyridine | 0 | 43 |
| 9 | 2-chloropyridine | 2.0 | 91 |
Reaction conditions: Tf2O (1.1 equiv), CH2Cl2, 45 °C, 2 h.
