Table 2.
Direct comparison of condensation reaction conditions.
| reaction conditions |
||||
|---|---|---|---|---|
| product | Tf2O (1.2 equiv) 2-ClPyr (This work)a | POCl3 (3.0 equiv) (Ref2a)b | Oxalyl Chloride (1.1 equiv) FeCl3 (Ref2d)c | Tf2O (5.0 equiv) DMAP (Ref2c)d |
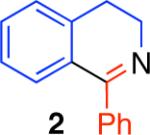 |
95% | 23% | 15% | 71% |
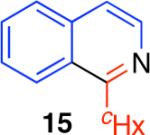 |
63% | 0% | 9% | 42% |
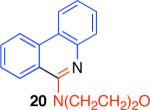 |
86% | 10% | 0% | 63% |
See Figure 1 for reaction conditions.
POCl3 (3.0 equiv), xylenes, 150 °C, 3 h.
1) Oxalyl chloride (1.1 equiv); FeCl3 (1.2 equiv), CH2Cl2, 23 °C, 12 h. 2) MeOH–H2SO4 (19:1), 65 °C, 1 h.
Tf2O (5.0 equiv), DMAP (3.0 equiv), CH2Cl2 23 °C, 16 h.
