Abstract
A major consequence of traumatic injury is the immunosuppression. Findings from previous studies suggest that the depression of immune functions is severe in young males, ovariectomized and aged females. In contrast, the immune functions in proestrus females following trauma-hemorrhage are maintained. Studies have also shown that the survival rate in proestrus females following trauma-hemorrhage and the induction of subsequent sepsis is significantly higher than in age-matched males and ovariectomized females. Furthermore, administration of female sex hormone 17β-estradiol in males and ovariectomized females after trauma-hemorrhage prevents the suppression of immune response. Thus, these findings suggest that sex hormones play a significant role in shaping the host response following trauma. This article reviews studies delineating the mechanism by which sex hormones regulate immune cell functions in the experimental model of trauma-hemorrhage. The findings from the studies reviewed in this article suggest that sex steroids can be synthesized by the immune cell. The findings further indicate that T cell and macrophages express receptors for androgen and estrogen. Since these cells are also the cells that produce cytokines, local synthesis of active steroids in these cells may become the significant factor in modulating their cytokine production.
Keywords: Injury, immune response, organ function, gender, estrogen, androgen
Introduction
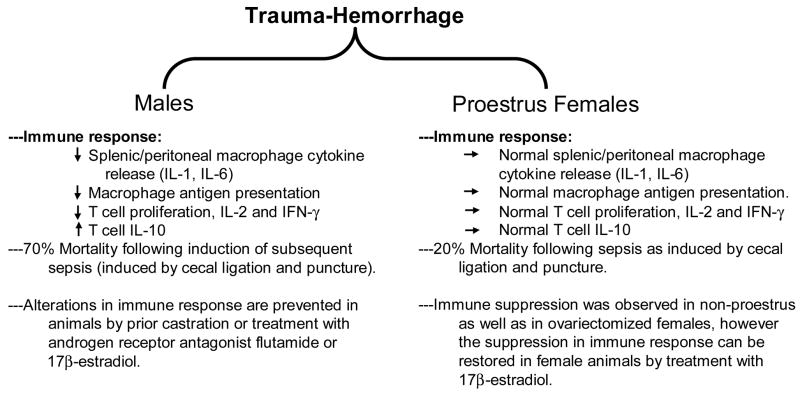
Trauma is not only the leading cause of deaths during the first three decades of life but it has an enormous socioeconomic impact 13;14. According to some estimates, trauma-related deaths and injuries contribute more to costs and loss of work time than cardiovascular diseases or malignancies 13;14. The most notable consequence of trauma is the development of immune suppression 2;17;19;37–39;57;61;69. Studies have shown that trauma/injury results in an inflammatory response which is characterized by aberrant production of cytokines, chemokines and other inflammatory mediators 2;6;11;15;17;19;45;61;69;76. This is accompanied with a profound suppression of the immune response. In addition, the functions of other organs including heart, lung and liver are also severely compromised 2;16;17;19;37–39;46;47;57;65. The primary function of the immune system is to maintain defense against the invasion of any deleterious foreign agents/particles including the infectious agents into the body. However in performing this function, the immune system has to maintain a balance so that it does not cause an undesired damage to the individual. This balance of the immune system is lost following severe hemorrhage and other traumatic injuries. Several lines of evidence suggest that excessive blood loss and accompanying hypoxia results in uncontrolled production of pro-inflammatory cytokines 2;16;17;19;37–39;57;65. Since a number of cells contribute to the production of these inflammatory mediators; studies were performed to determine post-injury immune cells functions using a number of experimental models of trauma. However, trauma is such a vast area and cannot be covered in one article. This article reviews findings from the studies performed following excessive blood loss. Furthermore, since both clinical and experiment findings suggest gender to be a major determinant in the outcome of trauma (Fig. 1) 2;6;9–11;15;17;17;19;19;24–26;46;47;55;61;66;69;76, the role of sex hormones on post-trauma immune pathogenesis will be discussed.
Figure 1. Gender Specific immune response following trauma-hemorrhage.
Sex hormones and macrophage functions following trauma-hemorrhage
Macrophage is an important cell of the innate immune system 28;61;68;69. They act immediately in response to any insult to the body including any kind of stress, trauma and invasion of foreign particles or pathogens. In case of particles/pathogens, macrophages phagocytose and destroy the undesirable foreign bodies that enter the body. In addition, they release a number of cytokines/chemokines which help in developing an effective immune defense against any foreign antigens. Since macrophages are the principal cell for the synthesis of the majority of inflammatory cytokines, a change in the release of cytokines by macrophages following trauma-hemorrhage is likely to influence the global release of inflammatory mediators. Macrophages also participate in developing the acquired or adaptive immune response by presenting antigen to T cell, and thus they act as antigen presenting cells. Several lines of evidence suggest that macrophage harvested from various tissue compartments following trauma-hemorrhage respond differently 42;75;83. In an effort to consolidate this idea, we performed a study in which macrophages were harvested from spleen, peritoneal cavity, lung and liver following trauma-hemorrhage 42;75;83. In addition, blood mononuclear cells (PBMC) were also harvested. PBMCs and macrophages thus harvested from various tissue organs were then evaluated for their ability to release IL-6 and TNF-α. Both IL-6 and TNF-α are the most common cytokines that are synthesized and released primarily by macrophages in response to injury and disease conditions. As summarized in Table 1, the findings from these studies suggest that PBMC, splenic and peritoneal macrophages exhibit a decreased IL-6 and TNF-α productive capacity following trauma-hemorrhage 42;75;83. The production of IL-6 and TNF-α by Kupffer cells and alveolar macrophage on the other hand was significantly increased following trauma-hemorrhage 33;41;86. However, the mechanism by which such compartmentalized immune cell responses occur following trauma-hemorrhage in males is not known.
Table 1.
Immune cell function in various tissue compartments following trauma-hemorrhage
| Peripheral Blood: |
| ↓PBMC IL-6, TNF-α |
| Spleen: |
| ↓Macrophage antigen presentation |
| ↓Macrophage IL-6 and TNF-α |
| Peritoneal Cavity: |
| ↓Macrophage antigen presentation |
| ↑Macrophage IL-6, TNF-α |
| Liver: |
| ↓Kupffer cells antigen presentation |
| ↑Kupffer cell IL-6 and TNF-α |
| Lung: |
| ↑Macrophage IL-6 and TNF-α |
Studies have also demonstrated that the alterations in macrophage functions following trauma-hemorrhage are gender specific 3;6;7. For example, males are shown to develop alteration in macrophage functions, whereas females in proestrus stage of estrus cycle do not (Fig. 1). However, ovariectomized female mice exhibit altered macrophage function similar to those observed in male mice following trauma-hemorrhage. However, treatment of male or ovariectomized mice with estrogen following trauma-hemorrhage prevents the alteration in macrophage functions. Furthermore, castration of male prior to trauma-hemorrhage also prevented alterations in macrophage functions that were observed in various compartment of the body following trauma-hemorrhage 3;5–7;17;18. Additionally, studies have also shown that administration of flutamide (androgen receptor antagonist) in males restores macrophage functions following trauma-hemorrhage similarly to those observed in normal animals. These findings suggest that sex steroids, 5α-dihydrotestosterone and 17β-estradiol play a critical role in the divergent immune responses in males and females following trauma-hemorrhage.
Sex hormones and dendritic cell functions following trauma-hemorrhage
Dendritic cell is another important cell of the immune system that plays a major role in bridging the innate and adaptive immune response. They are often referred as the professional antigen presenting cells. Dendritic cells were originally described by Steinman and Cohn 78 in 1973. Immature dendritic cells are located in tissues and continuously monitor the environment via the uptake of both particulate and soluble products. Once matured, they express high levels of MHC and other co-stimulatory molecules, including CD40, CD80, CD83, and CD86, on their surface 8;78. Mature dendritic cells also play a major role in T cell differentiation into Th1 and Th2. Dendritic cell maturation is accompanied with enhanced production of inflammatory cytokines and chemokines, reduced endocytic and phagocytic capacity. With the maturation, they also acquire migratory functions so that the antigen-loaded dendritic cells can move from the marginal zones to the T cell areas or from non-lymphoid to lymphoid tissues. Previous studies have shown that dendritic cells were depleted in the spleen of patients with sepsis 36. The findings from this study further indicated that the loss in splenic dendritic cell population in septic patients was significantly more than in trauma patients without sepsis 36. Additional findings suggest that the loss of dendritic cells during sepsis could result from an increased apoptosis 21;36;87. Furthermore, it has been reported that monocyte conversion to immature dendritic cell is impaired in trauma patients. Similar to septic patients, apoptosis was found to be the cause for depletion of dendritic cell in septic mice 21;36;87. Consistent with these studies, findings obtained in our laboratory at two hours after trauma-hemorrhage suggest a suppression in splenic dendritic cell antigen presenting ability and other co-stimulatory functions 44. This was accompanied with decreased MHC class II expression. Moreover, LPS-induced IL-12 production following trauma-hemorrhage was significantly decreased 44. While the precise mechanism of suppressed dendritic cell function following trauma-hemorrhage remains to be determined, it is likely that a decreases in MHC class II expression and IL-12 production following trauma-hemorrhage may contribute to suppressed dendritic cell antigen presentation. The suppression in dendritic cell antigen presentation could contribute to the host’s enhanced susceptibility to sepsis following trauma-hemorrhage. The role of sex hormones in altered dendritic cell functions following trauma-hemorrhage remains to be determined.
Sex hormones and T cell functions following trauma-hemorrhage
T cells are the major players in adaptive immune response and thus are critical to host defense. Previous studies from our laboratory (as summarized in Fig. 1) have demonstrated an association between T cell suppression and increased susceptibility to sepsis following trauma-hemorrhage 6;17–19;48;50;51;74;79;80. Thus, in order to maintain the immune system and avoid subsequent infection, it is important to prevent the suppression of T cell functions following trauma-hemorrhage. Additional studies from our laboratory have shown that splenic T cell proliferation and the production of Th1 cytokines (IL-2 and IFN-γ) were depressed in young males after trauma-hemorrhage (Fig 1). The release of Th2 cytokines IL-10 on the other hand was significantly elevated following trauma-hemorrhage compared to sham injured mice 6;17–19;48;50;51;74;79;80. Treatment of male animals with estrogen prevented the decrease in T cell functions. The suppression in T cell proliferation or in Th1 cytokines following trauma-hemorrhage was also not evident in pre-castrated males 6;17–19;48;50;51;74;79;80. Furthermore, studies have demonstrated that proestrus females, a stage of estrus cycle in which estrogen levels are high, did not show decrease in T cell proliferations and their production of IL-2 and IFN-γ compared to male mice following trauma-hemorrhage. Additionally, proestrus females had a significantly lower mortality following trauma-hemorrhage and induction of subsequent sepsis than male mice 6;17–19;48;50;51. Our previous studies also showed that administration of a single dose of 17β-estradiol following trauma-hemorrhage improved macrophage and lymphocyte functions 6;17–19;48;50;51;74. However, blockade of estrogen receptors by administration of EM-800 abolished the salutary effects of 17β-estradiol 6;17–19;48–51. These studies further corroborates that sex hormones play a major role in shaping the immune response following trauma-hemorrhage.
Immune cell and sex hormone synthesis
Several lines of evidence suggest that immune cells have the ability to synthesize the sex steroids 15;72;73. The synthesis of sex hormone is regulated at multiple steps 15;18;72;73;97;98. Cholesterol is the starting material for sex steroid synthesis. Studies have shown that cholesterol is converted to testosterone which is commonly found in both males and females 15;18;72;73;97;98. However, the subsequent conversion of testosterone into male 5α-dehydrotestosterone (5α-DHT) and into female hormone 17β-estradiol is regulated by two critical enzymes, 5α-reductase and aromatase, respectively. Testosterone in the presence of 5α-reductase is converted into male steroid 5α-DHT. In contrast, aromatase converts testosterone into female steroid 17β-estradiol. Thus, a slight change in the activity of 5α-reductase and aromatase will influence the production of male (5α-DHT) or female (17β-estradiol) sex hormones 15;18;72;73;97;98. Both 5α-reductase and aromatase along with other enzymes involved in sex steroid synthesis are present in immune cells including spleen and T cell 15;18;72;73;97. Previous studies from our laboratory have shown that trauma-hemorrhage influences the expression and activity of 5α-reductase and aromatase in the T cells 15;18;72–74;97;98. We found that in males, there was a significant increase in T cell 5α-reductase activity following trauma-hemorrhage 15;18;72–74;97;98. Aromatase activity in T cells of males on the other hand was relatively low and is not altered following trauma-hemorrhage. Thus, an increase in T cell 5α-reductase activity is likely to cause of increased synthesis of 5α-DHT by these cells. The increase in 5α-DHT is correlated with a decrease in T cell functions following trauma-hemorrhage 15;18;72–74;97;98.
In contrast to males, T cells in the proestrus females have high aromatase and low 5α-reductase activity following trauma-hemorrhage. This means the conversion of testosterone to 17β-estradiol is higher than 5α-DHT in proestrus females following trauma-hemorrhage 15;18;72–74;97;98. Because of high 17b-estrdiol levels, T cells from proestrus females maintain their functions following trauma-hemorrhage. These findings suggest that steroidogenic enzymes differ between males and proestrus females and that the trauma-hemorrhage further influences the activity of these enzymes which in turn may play a major role in altered immune cell functions under those conditions.
Immune cells sex hormone receptors expression
Almost all the cells, including the cells of the immune system, express receptors for both male (androgen) and female (estrogen) hormones 15;67. However, recent studies have indicated that the distribution of androgen and estrogen receptors is organ specific. Although no further major subdivision of androgen receptor (AR) has been reported, two major subtypes of estrogen receptors (ER), i.e., ER-α and ER-β, are reported to exist. Furthermore, several isoforms exists within each ER subtypes. For example, ER-α is further categorized into ER-αA, ER-αC, ER-αE, and ER-αF, and similarly ER-β into ER-β1, ER-β2, ER-β4, and ER-β5 15;18;54;67.
Recently, studies were performed determining the role of ERs in estrogen-mediated protection of organ functions following trauma-hemorrhage. The findings from those studies suggest the difference in the distribution of ER-α and -β in heart, liver, small intestine, and lung. ER-α mRNA expression was highest in the liver, whereas lung is predominantly rich in ER-β mRNA expression. Intestine on the other hand was found to express both ER-α and -β equally. Moreover, recent studies suggested a decrease in expression of ER-α and -β mRNA and protein in cardiomyocytes from males following trauma-hemorrhage 18;83;92–94. However, expression of cardiomyocytes AR mRNA and protein were not significantly different between the male trauma-hemorrhage and sham group 95. Although, these findings suggest that trauma-hemorrhage influences the ER expression in cardiomyocyte, it remains to be determined whether their distribution in other organs is also affected following trauma-hemorrhage. Thus, it is possible that the differences between the ER subtypes in tissue distribution may contribute to the selective action of ER agonists in different tissues 18;83;92–94. Studies have utilized ER-α and -β specific agonists, Propyl pyrazole triol (PPT) and diarylpropionitrile (DPN), to determine the role of ER-α and -β in protecting organ function following trauma-hemorrhage. PPT belongs to a series of tetrasubstituted pyrazole analogs and is the best characterized selective agonist for the ER-α subtype 6;15;18;43;59;60;64;77. PPT binds to ER-α with high affinity, displaying a 410-fold binding selectivity over ER-β 77. DPN, on the other hand, is an ER-β agonist and it can also bind to ER-α but its relative binding affinity for ER-β is 70-fold higher than ER-α. Furthermore, DPN has a 170-fold higher relative estrogenic potency in transcription assays with ER-β than ER-α 64. Our results provide evidence that following trauma-hemorrhage, estrogen-induced reduction of myeloperoxidase activity (MPO, an index of neutrophil infiltration) activity is mediated via ER-α activation in the liver, via ER-β activation in the lung, and via ER-α and ER-β in the small intestine 94. Those findings are consistent with ER mRNA expression in the liver, small intestine, and lung (i.e., ER-α mRNA expression is highest in the liver, and ER-β mRNA expression is greatest in the lung).
In a recent study, we examined which of the ER subtype mediates the salutary effects of 17β-estradiol on systemic inflammatory response/immune cell cytokine production in various tissues following trauma-hemorrhage 33–35;83. The findings suggested that 17β-estradiol or PPT administration following trauma-hemorrhage prevented the increase in plasma IL-6 and IL-10 levels. However the production of IL-6, IL-10 and TNF-α was found to be receptor specific. For example, IL-6 and TNF-α production by Kupffer cells increased following trauma-hemorrhage 33–35;83. However, splenic macrophages, alveolar macrophages, and peripheral blood mononuclear cells exhibit a decreased release of these cytokines following trauma-hemorrhage. The production of IL-10 on the other hand is increased in all macrophage populations. Administration of 17β-estradiol following trauma-hemorrhage prevented the alterations in cytokine production. However, with regard to specific receptor agonist, we found that ER-α agonist PPT had the same effects as 17β-estradiol on IL-6 and TNF-α production by Kupffer cells and splenic macrophages. ER-β agonist was effective on alveolar macrophages and peripheral blood mononuclear cells 83. Thus, ER subtypes may have tissue-specific roles in mediating the effects of 17β-estradiol on immune cell functions following trauma-hemorrhage. However, more studies are needed to confirm these findings.
In contrast to ER agonists, there are pharmacological agents which can block estrogen receptors 6;15;18;43;59;60;64;77. Among them, ICI 182,780 and EM-800 were used in many previous studies dealing with trauma 6;15;18;90. EM-800 blocks the transcriptional functions of ER α and β 58;64;88. ICI 182, 780 on the other hand inhibits estrogen binding to the receptor complex 6;15;18;90. These agents have been used to block estrogen effects following trauma-hemorrhage in some studies dealing with the mechanism of action of estradiol.
To delineate the role of male sex hormones, studies have used flutamide which is an androgen receptor antagonist. Flutamide blocks receptors of testosterone, 5α-dihydrotestosterone and 3α-androstenediol 6;15;18;90. Flutamide is a nonsteroidal agent and is known to inhibit androgen uptake or nuclear binding of the activated androgen receptor to nuclear response elements in the nucleus 3;4;6;7;15;53;90. Another common anti-androgenic compound is Bicalutamide (AstraZeneca). Bicalutamide, similar to flutamide is a non-steroidal and shares with flutamide its mechanism of action 6;15;27;53;90. This compound competitively inhibits the binding of androgen to its cytosolic receptor in target tissues and is used clinically in the treatment of prostate cancer.
Mechanism of action
Both androgen and estrogen receptors are localized in the cytoplasm and nucleus of the cell 6;15;22;43;53;67;90. Thus, both androgen and estrogen mediate their actions by activating the transcription factors and accordingly are expected to alter signaling at the nuclear level (i.e., the genomic mechanism). However, recent findings suggest that both genomic and non-genomic pathways are involved in mediating the effects of androgen and estrogen 18;20;22;31;32;62;67;82;89.
Androgen receptors
Findings from several studies suggest that the biological actions of male sex hormones are mediated via both nuclear and surface receptors. Androgens have been shown to induce the rapid activation of kinase-signaling cascades and modulate intracellular calcium levels 31;67;89. These effects are considered to be non-genomic because they are too rapid to involve changes in gene transcription. Furthermore, the effects are also shown in the presence of inhibitors of transcription and translation. Such non-genomic effects of androgens may occur through AR functioning in the cytoplasm to induce the MAPK signal cascade 31;67;89. However, sex steroids can also induce a non-genomic response within cells, a response that is not mediated through classical nuclear receptors, but rather initiated at the plasma membrane, presumably through unconventional surface receptors 18;31;67;82;89. Utilizing a cell-impermeable testosterone-BSA, studies have provided evidence for the presence of functional receptors of testosterone on the cell surface 18;31;67;89. Recent studies 31;89 have described interesting studies on the non-genomic action of androgens. They have shown that testosterone-induced increase in intracellular Ca2+ in macrophages and T cells is not inhibited by the AR-blocker cyproterone. Furthermore, it is also inducible by the plasma membrane impermeable ligand testosterone-BSA. The nature of this receptor remains to be investigated 31;89. Furthermore, while the physiological effect of non-genomic androgen action has yet to be determined, it is likely that it may ultimately contribute to regulation of transcription factor activity, including mediation of the transcriptional activity of AR.
Estrogen receptors
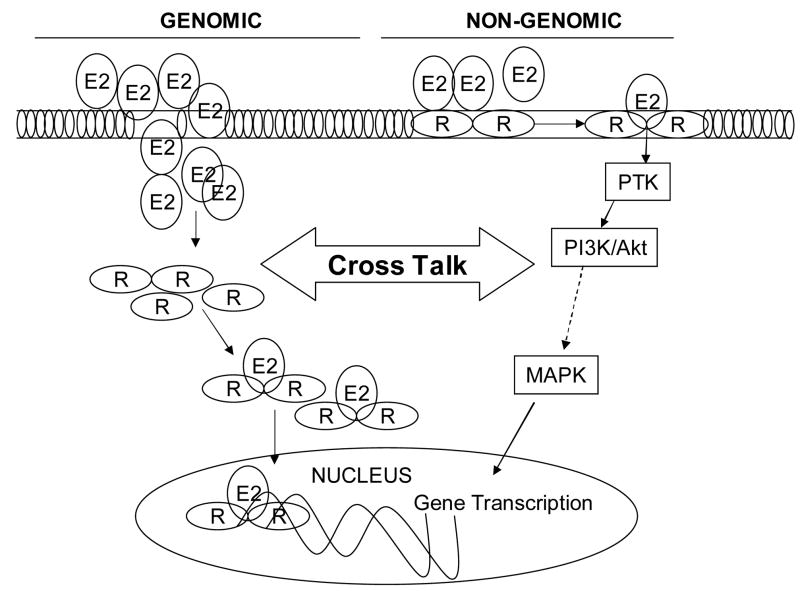
With regard to the genomic actions, studies have suggested that ER upon binding to estrogen becomes activated and dimerized (Fig. 3). The complex then translocates to the nucleus where it binds a specific target DNA sequence within the estrogen-responsive genes called an estrogen response elements (ERE) 18;20;31;32;62;67;82;89. Studies have also indicated that estrogen receptors which are not bound to estrogen can also bind to ERE consensus sequences and activate transcription. However, the interaction of the receptor with estrogen stabilizes dimerization and enhances its interaction with target sequences within estrogen-responsive genes. Besides 17β-estradiol, several other growth factors, including epidermal growth factor and insulin-like growth factor-1 can activate estrogen receptors and thus promote gene transactivation in the absence of estrogen 29;30;52;56.
The role of non-genomic pathways is also explored in estrogen actions using a cell-impermeable estrogen conjugated with BSA (E2-BSA). As depicted in Fig. 3, many of the signaling pathways (e.g., PLC/PKC; p38/MAPK; PI3k/AKT, NO, Ca2+) are turned on after stimulation of cells within E2-BSA 1;12;23;29;30. The non-genomic effects of estrogen can regulate different cellular processes, such as proliferation, survival, apoptosis, and differentiated functions in diverse cell types, including breast cancer cells 1;12;23;29;30. While ER-α and ER-β have been identified at the membrane, the nature of interaction of estrogen with the plasma membrane binding sites is currently under investigation. Furthermore the role of surface versus nuclear receptors in mediating the salutary effect of estrogen following trauma-hemorrhage on immune cell functions is also under investigation.
While the role of surface versus nuclear receptor in the regulation of gender-specific immune responses following trauma-hemorrhage remains to be investigated, few studies have evaluated the relative contribution of cell surface and nuclear membrane receptors in estrogen-mediated protection of other organ function following trauma-hemorrhage. The findings from these studies suggest that male rats treated with E2-BSA displayed improvement in cardiac functions following trauma-hemorrhage 91. These findings further suggested that biologic effects of E2-BSA on cardiac function are receptor-dependent since the administration of a selective estrogen receptor antagonist ICI 182,780 along with E2-BSA abolished the E2-BSA-induced cardioprotection following trauma-hemorrhage. However, the restoration of cardiac function following E2-BSA treatment was not complete as compared to the rats treated with estrogen alone. Thus, both surface and nuclear estrogen receptors are likely required for full restoration of cardiac function following trauma-hemorrhage. Our studies have also demonstrated that PI3K/Akt pathway plays major role in mediating the non-genomic effects of E2 on cardiac functions. Similar findings were obtained by other investigators supporting the role of PI3K/Akt signaling in non-genomic effects of E2 1;12;23;29;30. However, it should be noted that although the genomic and non-genomic actions of estrogen are interdependent as shown in Fig. 3, both genomic and non-genomic pathway work synergistically in the regulation of cell functions whether it is a cardiomyocyte or an immune cell.
In addition to the classical ERs, the role of G protein-coupled receptor 30 (GPR30) is also demonstrated in mediating estrogen actions 32;40;70;71. Studies have indicated that estrogen can also mediate its effect independently of estrogen receptors via GPR30. In this regards, GPR30 is a novel ER and has been suggested as a candidate for triggering a broad range of E2-mediated signaling 71. GPR30 is located on the cell membrane and presents an alternative to the classical ERs. Estrogen binds directly to GPR30 and mediate downstream signaling cascade. Using this pathway, E2 is shown to promptly activate the adenylate cyclase and induces cAMP/PKA signaling pathway in human keratinocytes and breast cancer cells GPR30. Furthermore, the overexpression of GPR30 in breast cancer cells restores the activation of adenylyl cyclase by E2 and suppression of GPR30 expression with antisense oligonucleotides or siRNA prevents E2-mediated cAMP-dependent signaling in keratinocytes and in SKBR3 breast cancer cells that lack ERα and ERβ. Recent findings from our laboratory have also shown that E2 utilizes GPR30 in activating PKA pathway in isolated hepatocytes 40. However, more studies are needed to fully understand the role of GPR30 in E2 mediated effects on immune cell functions following trauma-hemorrhage.
Estrogen and heat shock proteins
In addition, estrogen can also induce the expression of heat shock proteins (HSP). Studies have shown that HSP plays a role in the preservation of organ function under adverse circulatory conditions 56;81;84;85;93;96. For example, HSP60 and HSP70 serve as molecular chaperones and maintain protein structures under stress conditions. HSP60 is localized in the mitochondria and is reported to be helpful in maintaining electron chain integrity 56. HSP32, also referred as heme oxygenase-1 (HO-1), is shown to play a protective role following trauma-hemorrhage 84;85;93;96. Studies have indicated that HO-1 participates in heme elimination. The accumulation of free heme under hypoxic conditions becomes toxic and thus in order to maintain organ functions, its removal from the cellular milieu becomes necessary. Bilirubin, a product of HSP32 enzyme activity, is shown to have potent antioxidant activity. In a recent study, we found that estrogen administration upregulates HSP32 following trauma-hemorrhage. Furthermore, HSP32 upregulation inhibits the expression of adhesion molecules and prevent subsequent leukocyte-endothelial cell interactions under those conditions 84;85;93;96. Other investigators have shown that an upregulation in HSP32 protects mitochondrial function and prevents ATP-depletion after oxidative stress. Heat shock proteins are also known to regulate the process of programmed cell death or apoptosis 56;63. Thus, the HSP protect cells via multiple mechanisms. Although the mechanism by which estrogen regulates HSP remains to be established, studies have indicated that the synthesis of HSP is regulated by a family of heat shock factors (HSF). There are four HSF that have been identified. Among them HSF-1 is shown to play a predominant role in the regulation of HSP in response to trauma and other adverse conditions such as ischemia and hypoxia 29;30;52;56;63;93.
Conclusion
A major consequence of trauma-hemorrhage is the suppression of the immune cell functions. The findings reviewed in this article suggest that the generation of immune response to trauma is gender dimorphic and sex steroids play a decisive role in the depression or maintenance of immune functions following injury. These findings suggest that trauma-hemorrhage results in profound alterations in immune functions in males and ovariectomized females. However, they are maintained in pre-castrated males and proestrus females. The immune responses in males and ovariectomized females are also protected in animals treated with estrogen following trauma-hemorrhage. The beneficial effects of female sex steroid 17β-estradiol and the deleterious effects of male hormones 5α-dihydrotestosterone in immune functions has been demonstrated with the use of gonadectomized animals as well as by treating animals with hormonal agonists and antagonists following trauma-hemorrhage. Since steroids are involved in the regulation of immune cells functions, modulation of such cells early after trauma with selective estrogen agonists may be a useful approach for maintaining the immune functions under those conditions.
In summary, while many studies suggest the beneficial effect of estrogen following trauma, more studies are needed to fully understand the precise mechanism by which estrogen mediates its beneficial effects following trauma-hemorrhage in females and males, respectively.
Figure 2. Genomic and nongenomic actions of estrogen.
E2, estrogen; R, estrogen receptor; PTK, protein tyrosine kinases; PI3K phosphatidylinositol 3-kinase, MAPK, mitogen activated protein kinase.
Acknowledgments
This work was supported by National Institutes of Health grants AA015979-01A1 (MAC), R37 GM39519 and R01 GM37127 (IHC).
Footnotes
Conflict of interest statement
The authors have neither financial nor proprietary interest in the subject matter or materials discussed in the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Acconcia F, Kumar R. Signaling regulation of genomic and nongenomic functions of estrogen receptors. Cancer Lett. 2006;238:1–14. doi: 10.1016/j.canlet.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 2.Ananthakrishnan P, Cohen DB, Xu DZ, et al. Sex hormones modulate distant organ injury in both a trauma/hemorrhagic shock model and a burn model. Surgery. 2005;137:56–65. doi: 10.1016/j.surg.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 3.Angele MK, Ayala A, Cioffi WG, et al. Testosterone: the culprit for producing splenocyte immune depression after trauma hemorrhage. Am J Physiol. 1998;274:C1530–C1536. doi: 10.1152/ajpcell.1998.274.6.C1530. [DOI] [PubMed] [Google Scholar]
- 4.Angele MK, Knöferl MW, Ayala A, et al. Testosterone and estrogen differently effect th1 and th2 cytokine release following trauma-haemorrhage. Cytokine. 2001;16:22–30. doi: 10.1006/cyto.2001.0945. [DOI] [PubMed] [Google Scholar]
- 5.Angele MK, Knoferl MW, Schwacha MG, et al. Sex steroids regulate pro- and anti-inflammatory cytokine release by macrophages after trauma-hemorrhage. Am J Physiol. 1999;277:C35–C42. doi: 10.1152/ajpcell.1999.277.1.C35. [DOI] [PubMed] [Google Scholar]
- 6.Angele MK, Schwacha MG, Ayala A, Chaudry IH. Effect of gender and sex hormones on immune responses following shock. Shock. 2000;14:81–90. doi: 10.1097/00024382-200014020-00001. [DOI] [PubMed] [Google Scholar]
- 7.Angele MK, Wichmann MW, Ayala A, et al. Testosterone receptor blockade after hemorrhage in males. Restoration of the depressed immune functions and improved survival following subsequent sepsis. Arch Surg. 1997;132:1207–1214. doi: 10.1001/archsurg.1997.01430350057010. [DOI] [PubMed] [Google Scholar]
- 8.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 9.Barrow RE, Herndon DN. Incidence of mortality in boys and girls after severe thermal burns. Surgery. 1990;170:295–298. [PubMed] [Google Scholar]
- 10.Barrow RE, Przkora R, Hawkins HK, et al. Mortality related to gender, age, sepsis, and ethnicity in severely burned children. Shock. 2005;23:485–487. [PubMed] [Google Scholar]
- 11.Baue AE, Durham R, Faist E. Systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), multiple organ failure (MOF): are we winning the battle? Shock. 1998;10:79–89. doi: 10.1097/00024382-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 13.Carrico CJ, Holcomb JB, Chaudry IH. Scientific priorities and strategic planning for resuscitation research and life saving therapy following traumatic injury: report of the PULSE Trauma Work Group. Acad Emerg Med. 2002;9:621–626. doi: 10.1111/j.1553-2712.2002.tb02303.x. [DOI] [PubMed] [Google Scholar]
- 14.Carrico CJ, Holcomb JB, Chaudry IH. Scientific priorities and strategic planning for resuscitation research and life saving therapy following traumatic injury: report of the PULSE Trauma Work Group. Post Resuscitative and Initial Utility of Life Saving Efforts. Shock. 2002;17:165–168. doi: 10.1097/00024382-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Chaudry IH, Samy TS, Schwacha MG, et al. Endocrine targets in experimental shock. J Trauma. 2003;54:S118–S125. doi: 10.1097/01.TA.0000064511.14322.F1. [DOI] [PubMed] [Google Scholar]
- 16.Chen T, Zamora R, Zuckerbraun B, Billiar TR. Role of nitric oxide in liver injury. Curr Mol Med. 2003;3:519–526. doi: 10.2174/1566524033479582. [DOI] [PubMed] [Google Scholar]
- 17.Choudhry MA, Bland KI, Chaudry IH. Gender and susceptibility to sepsis following trauma. Endocr Metab Immune Disord Drug Targets. 2006;6:127–135. doi: 10.2174/187153006777442422. [DOI] [PubMed] [Google Scholar]
- 18.Choudhry MA, Chaudry IH. Gender dimorphism and use of sex steroid/receptor antagonist after trauma. In: Welsh CJ, Meagher MW, Sternberg EM, editors. Neural and Neuroendocrine Mechanisms in Host Defense and Autoimmunity. New York: Springer; 2006. pp. 101–121. [Google Scholar]
- 19.Choudhry MA, Schwacha MG, Hubbard WJ, et al. Gender differences in acute response to trauma-hemorrhage. Shock. 2005;24 Suppl 1:101–106. doi: 10.1097/01.shk.0000191341.31530.5e. [DOI] [PubMed] [Google Scholar]
- 20.Dahlman-Wright K, Cavailles V, Fuqua SA, et al. International Union of Pharmacology. LXIV. Estrogen receptors. Pharmacol Rev. 2006;58:773–781. doi: 10.1124/pr.58.4.8. [DOI] [PubMed] [Google Scholar]
- 21.Ding Y, Chung CS, Newton S, et al. Polymicrobial sepsis induces divergent effects on splenic and peritoneal dendritic cell function in mice. Shock. 2004;22:137–144. doi: 10.1097/01.shk.0000131194.80038.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Disilvio L, Jameson J, Gamie Z, et al. In vitro evaluation of the direct effect of estradiol on human osteoblasts (HOB) and human mesenchymal stem cells (h-MSCs) Injury. 2006;37:S33–S42. doi: 10.1016/j.injury.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 23.Driggers PH, Segars JH. Estrogen action and cytoplasmic signaling pathways. Part II: the role of growth factors and phosphorylation in estrogen signaling. Trends Endocrinol Metab. 2002;13:422–427. doi: 10.1016/s1043-2760(02)00634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenmenger SJ, Wichmann MW, Angele P, et al. Differences in the expression of LPS-receptors are not responsible for the sex-specific immune response after trauma and hemorrhagic shock. Cell Immunol. 2004;230:17–22. doi: 10.1016/j.cellimm.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Frantz MC, Prix NJ, Wichmann MW, et al. Dehydroepiandrosterone restores depressed peripheral blood mononuclear cell function following major abdominal surgery via the estrogen receptors. Crit Care Med. 2005;33:1779–1786. doi: 10.1097/01.ccm.0000172278.91959.38. [DOI] [PubMed] [Google Scholar]
- 26.Frink M, Pape HC, van Griensven M, et al. Influence of sex and age on MODS and cytokines after multiple injuries. Shock. 2007;27:151–156. doi: 10.1097/01.shk.0000239767.64786.de. [DOI] [PubMed] [Google Scholar]
- 27.Furr BJ. The development of Casodex (bicalutamide): preclinical studies. Eur Urol. 1996;29:83–95. doi: 10.1159/000473846. [DOI] [PubMed] [Google Scholar]
- 28.Gomez CR, Boehmer ED, Kovacs EJ. The aging innate immune system. Curr Opin Immunol. 2005;17:457–462. doi: 10.1016/j.coi.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 29.Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001;276:36869–36872. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- 30.Haynes MP, Russell KS, Bender JR. Molecular mechanisms of estrogen actions on the vasculature. J Nucl Cardiol. 2000;7:500–508. doi: 10.1067/mnc.2000.109958. [DOI] [PubMed] [Google Scholar]
- 31.Heinlein CA, Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol. 2002;16:2181–2187. doi: 10.1210/me.2002-0070. [DOI] [PubMed] [Google Scholar]
- 32.Hewitt SC, Deroo BJ, Korach KS. Signal transduction. A new mediator for an old hormone? Science. 2005;307:1572–1573. doi: 10.1126/science.1110345. [DOI] [PubMed] [Google Scholar]
- 33.Hildebrand F, Hubbard WJ, Choudhry MA, et al. Kupffer cells and their mediators: the culprits in producing distant organ damage after trauma-hemorrhage. Am J Pathol. 2006;169:784–794. doi: 10.2353/ajpath.2006.060010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hildebrand F, Hubbard WJ, Choudhry MA, et al. Are the protective effects of 17{beta}-estradiol on splenic macrophages and splenocytes after trauma-hemorrhage mediated via estrogen-receptor (ER)-{alpha} or ER-{beta}? J Leukoc Biol. 2006;79:1173–1180. doi: 10.1189/jlb.0106029. [DOI] [PubMed] [Google Scholar]
- 35.Hildebrand F, Hubbard WJ, Choudhry MA, et al. Effects of 17{beta}-estradiol and flutamide on inflammatory response and distant organ damage following trauma-hemorrhage in metestrus females. J Leukoc Biol. 2006;80:759–765. doi: 10.1189/jlb.0406254. [DOI] [PubMed] [Google Scholar]
- 36.Hotchkiss RS, Tinsley KW, Swanson PE, et al. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol. 2002;168:2493–2500. doi: 10.4049/jimmunol.168.5.2493. [DOI] [PubMed] [Google Scholar]
- 37.Hoyt DB. A clinical review of bleeding dilemmas in trauma. Semin Hematol. 2004;41:40–43. doi: 10.1053/j.seminhematol.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Hoyt DB, Junger WG, Loomis WH, Liu FC. Effects of trauma on immune cell function: impairment of intracellular calcium signaling. Shock. 1994;2:23–28. doi: 10.1097/00024382-199407000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Hoyt DB, Ozkan AN, Frevert J, et al. Alteration in Ca2+ homeostasis by a trauma peptide. J Surg Res. 1991;51:477–483. doi: 10.1016/0022-4804(91)90168-l. [DOI] [PubMed] [Google Scholar]
- 40.Hsieh YC, Yu HP, Frink M, et al. GPR30-dependent PKA pathway is critical in non-genomic effects of estrogen in attenuating liver injury following trauma-hemorrhage. Am J Pathol. 2007 doi: 10.2353/ajpath.2007.060883. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jarrar D, Kuebler JF, Rue LW, III, et al. Alveolar macrophage activation after trauma-hemorrhage and sepsis is dependent on NF-kappaB and MAPK/ERK mechanisms. Am J Physiol Lung Cell Mol Physiol. 2002;283:L799–L805. doi: 10.1152/ajplung.00465.2001. [DOI] [PubMed] [Google Scholar]
- 42.Kang SC, Matsutani T, Choudhry MA, et al. Are the immune responses different in middle-aged and young mice following bone fracture, tissue trauma and hemorrhage? Cytokine. 2004;26:223–230. doi: 10.1016/j.cyto.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor transcription and transactivation: Estrogen receptor alpha and estrogen receptor beta: regulation by selective estrogen receptor modulators and importance in breast cancer. Breast Cancer Res. 2000;2:335–344. doi: 10.1186/bcr78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kawasaki T, Hubbard WJ, Choudhry MA, et al. Trauma-hemorrhage induces depressed splenic dendritic cell functions in mice. J Immunol. 2006;177:4514–4520. doi: 10.4049/jimmunol.177.7.4514. [DOI] [PubMed] [Google Scholar]
- 45.Keel M, Trentz O. Pathophysiology of polytrauma. Injury. 2005;36:691–709. doi: 10.1016/j.injury.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 46.Kher A, Meldrum KK, Wang M, et al. Cellular and molecular mechanisms of sex differences in renal ischemia-reperfusion injury. Cardiovasc Res. 2005;67:594–603. doi: 10.1016/j.cardiores.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 47.Kher A, Wang M, Tsai BM, et al. Sex differences in the myocardial inflammatory response to acute injury. Shock. 2005;23:1–10. doi: 10.1097/01.shk.0000148055.12387.15. [DOI] [PubMed] [Google Scholar]
- 48.Knoferl MW, Angele MK, Diodato MD, et al. Female sex hormones regulate macrophage function after trauma-hemorrhage and prevent increased death rate from subsequent sepsis. Ann Surg. 2002;235:105–112. doi: 10.1097/00000658-200201000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knoferl MW, Angele MK, Schwacha MG, et al. Immunoprotection in proestrus females following trauma-hemorrhage: the pivotal role of estrogen receptors. Cell Immunol. 2003;222:27–34. doi: 10.1016/s0008-8749(03)00081-9. [DOI] [PubMed] [Google Scholar]
- 50.Knoferl MW, Angele MK, Schwacha MG, et al. Preservation of splenic immune functions by female sex hormones after trauma-hemorrhage. Crit Care Med. 2002;30:888–893. doi: 10.1097/00003246-200204000-00029. [DOI] [PubMed] [Google Scholar]
- 51.Knoferl MW, Jarrar D, Angele MK, et al. 17 beta-Estradiol normalizes immune responses in ovariectomized females after trauma-hemorrhage. Am J Physiol Cell Physiol. 2001;281:C1131–C1138. doi: 10.1152/ajpcell.2001.281.4.C1131. [DOI] [PubMed] [Google Scholar]
- 52.Knowlton AA, Sun L. Heat-shock factor-1, steroid hormones, and regulation of heat-shock protein expression in the heart. Am J Physiol Heart Circ Physiol. 2001;280:H455–H464. doi: 10.1152/ajpheart.2001.280.1.H455. [DOI] [PubMed] [Google Scholar]
- 53.Kolvenbag GJ, Nash A. Bicalutamide dosages used in the treatment of prostate cancer. Prostate. 1999;39:47–53. doi: 10.1002/(sici)1097-0045(19990401)39:1<47::aid-pros8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 54.Kos M, Denger S, Reid G, Gannon F. Upstream open reading frames regulate the translation of the multiple mRNA variants of the estrogen receptor alpha. J Biol Chem. 2002;277:37131–37138. doi: 10.1074/jbc.M206325200. [DOI] [PubMed] [Google Scholar]
- 55.Kovacs EJ, Messingham KA, Gregory MS. Estrogen regulation of immune responses after injury. Mol Cell Endocrinol. 2002;193:129–135. doi: 10.1016/s0303-7207(02)00106-5. [DOI] [PubMed] [Google Scholar]
- 56.Latchman DS. Heat shock proteins and cardiac protection. Cardiovasc Res. 2001;51:637–646. doi: 10.1016/s0008-6363(01)00354-6. [DOI] [PubMed] [Google Scholar]
- 57.Lederer JA, Rodrick ML, Mannick JA. The effects of injury on the adaptive immune response. Shock. 1999;11:153–159. doi: 10.1097/00024382-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 58.Luo S, Souria A, Labrie C, et al. Effect of twenty-four-week treatment with the antiestrogen EM-800 on estrogen-sensitive parameters in intact and ovariectomized mice. Endocrinology. 1998;139:2645–2656. doi: 10.1210/endo.139.5.5994. [DOI] [PubMed] [Google Scholar]
- 59.Maret A, Coudert JD, Garidou L, et al. Estradiol enhances primary antigen-specific CD4 T cell responses and Th1 development in vivo. Essential role of estrogen receptor alpha expression in hematopoietic cells. Eur J Immunol. 2003;33:512–521. doi: 10.1002/immu.200310027. [DOI] [PubMed] [Google Scholar]
- 60.Martel C, Labrie C, Belanger A, et al. Comparison of the effects of the new orally active antiestrogen EM-800 with ICI 182 780 and toremifene on estrogen-sensitive parameters in the ovariectomized mouse. Endocrinology. 1998;139:2486–2492. doi: 10.1210/endo.139.5.5968. [DOI] [PubMed] [Google Scholar]
- 61.Maung AA, Fujimi S, Miller ML, et al. Enhanced TLR4 reactivity following injury is mediated by increased p38 activation. J Leukoc Biol. 2005;78:565–573. doi: 10.1189/jlb.1204698. [DOI] [PubMed] [Google Scholar]
- 62.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 63.Meng X, Harken AH. The interaction between Hsp70 and TNF-alpha expression: a novel mechanism for protection of the myocardium against post-injury depression. Shock. 2002;17:345–353. doi: 10.1097/00024382-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 64.Meyers MJ, Sun J, Carlson KE, et al. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- 65.Mollen KP, Anand RJ, Tsung A, et al. Emerging paradigm: toll-like receptor 4-sentinel for the detection of tissue damage. Shock. 2006;26:430–437. doi: 10.1097/01.shk.0000228797.41044.08. [DOI] [PubMed] [Google Scholar]
- 66.Oberholzer A, Keel M, Zellweger R, et al. Incidence of septic complications and multiple organ failure in severely injured patients is sex specific. J Trauma. 2000;48:932–937. doi: 10.1097/00005373-200005000-00019. [DOI] [PubMed] [Google Scholar]
- 67.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286:R233–R249. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- 68.Plackett TP, Boehmer ED, Faunce DE, Kovacs EJ. Aging and innate immune cells. J Leukoc Biol. 2004;76:291–299. doi: 10.1189/jlb.1103592. [DOI] [PubMed] [Google Scholar]
- 69.Purcell EM, Dolan SM, Kriynovich S, et al. Burn injury induces an early activation response by lymph node CD4+ T cells. Shock. 2006;25:135–140. doi: 10.1097/01.shk.0000190824.51653.32. [DOI] [PubMed] [Google Scholar]
- 70.Qiu J, Bosch MA, Tobias SC, et al. A G-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci. 2006;26:5649–5655. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Revankar CM, Cimino DF, Sklar LA, et al. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 72.Samy TS, Knöferl MW, Zheng R, et al. Divergent immune responses in male and female mice after trauma-hemorrhage: dimorphic alterations in T lymphocyte steroidogenic enzyme activities. Endocrinology. 2001;142:3519–3529. doi: 10.1210/endo.142.8.8322. [DOI] [PubMed] [Google Scholar]
- 73.Samy TS, Zheng R, Matsutani T, et al. Mechanism for normal splenic T lymphocyte functions in proestrus females after trauma: enhanced local synthesis of 17beta-estradiol. Am J Physiol Cell Physiol. 2003;285:C139–C149. doi: 10.1152/ajpcell.00058.2003. [DOI] [PubMed] [Google Scholar]
- 74.Schneider CP, Nickel EA, Samy TS, et al. The aromatase inhibitor, 4-hydroxyandrostenedione, restores immune responses following trauma-hemorrhage in males and decreases mortality from subsequent sepsis. Shock. 2000;14:347–353. doi: 10.1097/00024382-200014030-00019. [DOI] [PubMed] [Google Scholar]
- 75.Shih-Ching K, Choudhry MA, Matsutani T, et al. Splenectomy differentially influences immune responses in various tissue compartments of the body. Cytokine. 2004;28:101–108. doi: 10.1016/j.cyto.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 76.Shukla A, Hashiguchi N, Chen Y, et al. Osmotic regulation of cell function and possible clinical applications. Shock. 2004;21:391–400. doi: 10.1097/00024382-200405000-00001. [DOI] [PubMed] [Google Scholar]
- 77.Stauffer SR, Coletta CJ, Tedesco R, et al. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- 78.Steinman RM, Cohn ZA. Pillars article: identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]; J Immunol. 2007;178:5–25. [Google Scholar]
- 79.Stephan RN, Kupper TS, Geha AS, et al. Hemorrhage without tissue trauma produces immunosuppression and enhances susceptibility to sepsis. Arch Surg. 1987;122:62–68. doi: 10.1001/archsurg.1987.01400130068010. [DOI] [PubMed] [Google Scholar]
- 80.Stephan RN, Mitsuyoski S, Conrad PJ, et al. Depressed antigen presentation function and membrane interleukin- 1 activity of peritoneal macrophages after laparotomy. Surgery. 1987;102:147–154. [PubMed] [Google Scholar]
- 81.Su F, Nguyen ND, Wang Z, et al. Fever control in septic shock: beneficial or harmful? Shock. 2005;23:516–520. [PubMed] [Google Scholar]
- 82.Suzuki T, Shimizu T, Yu HP, et al. 17b-estradiol administration following trauma-hemorrhage prevents the increase in Kupffer cell cytokine production and MAPK activation predominantly via estrogen receptor-a. Surgery. 2006;140:141–148. doi: 10.1016/j.surg.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 83.Suzuki T, Shimizu T, Yu HP, et al. Tissue compartment-specific role of estrogen receptor subtypes in immune cell cytokine production following trauma-hemorrhage. J Appl Physiol. 2007;102:163–168. doi: 10.1152/japplphysiol.00964.2006. [DOI] [PubMed] [Google Scholar]
- 84.Szalay L, Shimizu T, Schwacha MG, et al. Mechanism of salutary effects of estradiol on organ function after trauma-hemorrhage: upregulation of heme oxygenase. Am J Physiol Heart Circ Physiol. 2005;289:H92–H98. doi: 10.1152/ajpheart.01247.2004. [DOI] [PubMed] [Google Scholar]
- 85.Szalay L, Shimizu T, Suzuki T, et al. Estradiol improves cardiac and hepatic function after trauma-hemorrhage: role of enhanced heat shock protein expression. Am J Physiol Regul Integr Comp Physiol. 2006;290:R812–R818. doi: 10.1152/ajpregu.00658.2005. [DOI] [PubMed] [Google Scholar]
- 86.Thobe BM, Hildebrand F, Frink M, et al. The role of MAPK in Kupffer cell Toll like receptor (TLR) 2-, 4- and 9-mediated signaling following trauma hemorrhage. J Cell Physiol. 2006;210:667–675. doi: 10.1002/jcp.20860. [DOI] [PubMed] [Google Scholar]
- 87.Tinsley KW, Grayson MH, Swanson PE, et al. Sepsis induces apoptosis and profound depletion of splenic interdigitating and follicular dendritic cells. J Immunol. 2003;171:909–914. doi: 10.4049/jimmunol.171.2.909. [DOI] [PubMed] [Google Scholar]
- 88.Tremblay A, Tremblay GB, Labrie F, Giguere V. EM-800, a novel antiestrogen, acts as a pure antagonist of the transcriptional functions of estrogen receptors alpha and beta. Endocrinology. 1998;139:111–118. doi: 10.1210/endo.139.1.5702. [DOI] [PubMed] [Google Scholar]
- 89.Wunderlich F, Benten WP, Lieberherr M, et al. Testosterone signaling in T cells and macrophages. Steroids. 2002;67:535–538. doi: 10.1016/s0039-128x(01)00175-1. [DOI] [PubMed] [Google Scholar]
- 90.Yokoyama Y, Schwacha MG, Samy TS, et al. Gender dimorphism in immune responses following trauma and hemorrhage. Immunol Res. 2002;26:63–76. doi: 10.1385/ir:26:1-3:063. [DOI] [PubMed] [Google Scholar]
- 91.Yu HP, Hsieh YC, Suzuki T, et al. The PI3K/Akt pathway mediates the non genomic cardio protective effects of estrogen following trauma-hemorrhage. Ann Surg. 2007 doi: 10.1097/01.sla.0000254417.15591.88. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu HP, Hsieh YC, Suzuki T, et al. Salutary effects of estrogen receptor-beta agonist on lung injury after trauma-hemorrhage. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1004–L1009. doi: 10.1152/ajplung.00504.2005. [DOI] [PubMed] [Google Scholar]
- 93.Yu HP, Shimizu T, Choudhry MA, et al. Mechanism of cardioprotection following trauma-hemorrhagic shock by a selective estrogen receptor-beta agonist: up-regulation of cardiac heat shock factor-1 and heat shock proteins. J Mol Cell Cardiol. 2006;40:185–194. doi: 10.1016/j.yjmcc.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 94.Yu HP, Shimizu T, Hsieh YC, et al. Tissue-specific expression of estrogen receptors and their role in the regulation of neutrophil infiltration in various organs following trauma-hemorrhage. J Leukoc Biol. 2006;79:963–970. doi: 10.1189/jlb.1005596. [DOI] [PubMed] [Google Scholar]
- 95.Yu HP, Yang S, Choudhry MA, et al. Mechanism responsible for the salutary effects of flutamide on cardiac performance after trauma-hemorrhagic shock: Upregulation of cardiomyocyte estrogen receptors. Surgery. 2005;138:85–92. doi: 10.1016/j.surg.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 96.Yu HP, Yang S, Hsieh YC, et al. Maintenance of lung myeloperoxidase activity in proestrus females after trauma-hemorrhage: upregulation of heme oxygenase-1. Am J Physiol Lung Cell Mol Physiol. 2006;291:L400–L406. doi: 10.1152/ajplung.00537.2005. [DOI] [PubMed] [Google Scholar]
- 97.Zheng R, Samy TSA, Schneider CP, et al. Decreased 5 alpha-dihydrotestosterone catabolism suppresses T lymphocyte functions in males after trauma-hemorrhage. American Journal of Physiology-Cell Physiology. 2002;282:C1332–C1338. doi: 10.1152/ajpcell.00560.2001. [DOI] [PubMed] [Google Scholar]
- 98.Zoppi S, Lechuga M, Motta M. Selective inhibition of the 5 alpha-reductase of the rat epididymis. J Steroid Biochem Mol Biol. 1992;42:509–514. doi: 10.1016/0960-0760(92)90263-i. [DOI] [PubMed] [Google Scholar]