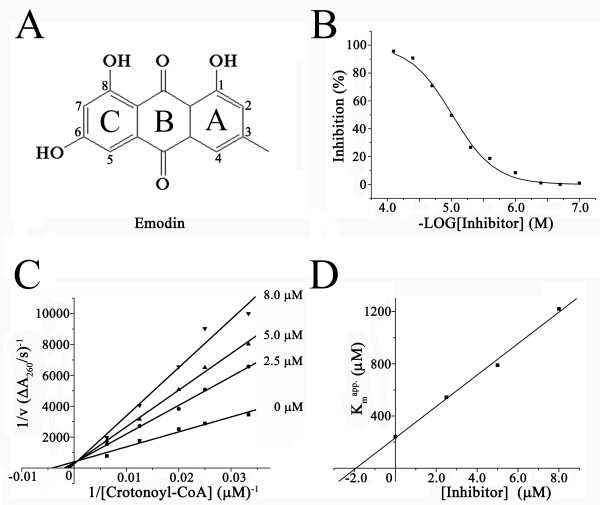
Figure 1.
(A) Chemical structure of Emodin. The three rings are named and their positions are numbered according to the nomenclature. (B) Dose-response curves for enzyme inhibition (IC50 = 9.70 ± 1.0 μM). (C) Kinetic analysis of Emodin inhibition against HpFabZ. The panel shows the representative double reciprocal plots of 1/V vs 1/[Substrate] at different inhibitor concentrations. The lines intercept on the 1/V axis, indicating that Emodin is a competitive inhibitor for the substrate crotonoyl-CoA. (D) Secondary plot of Km. The inhibition constant Ki is 1.9 ± 0.3 μM.