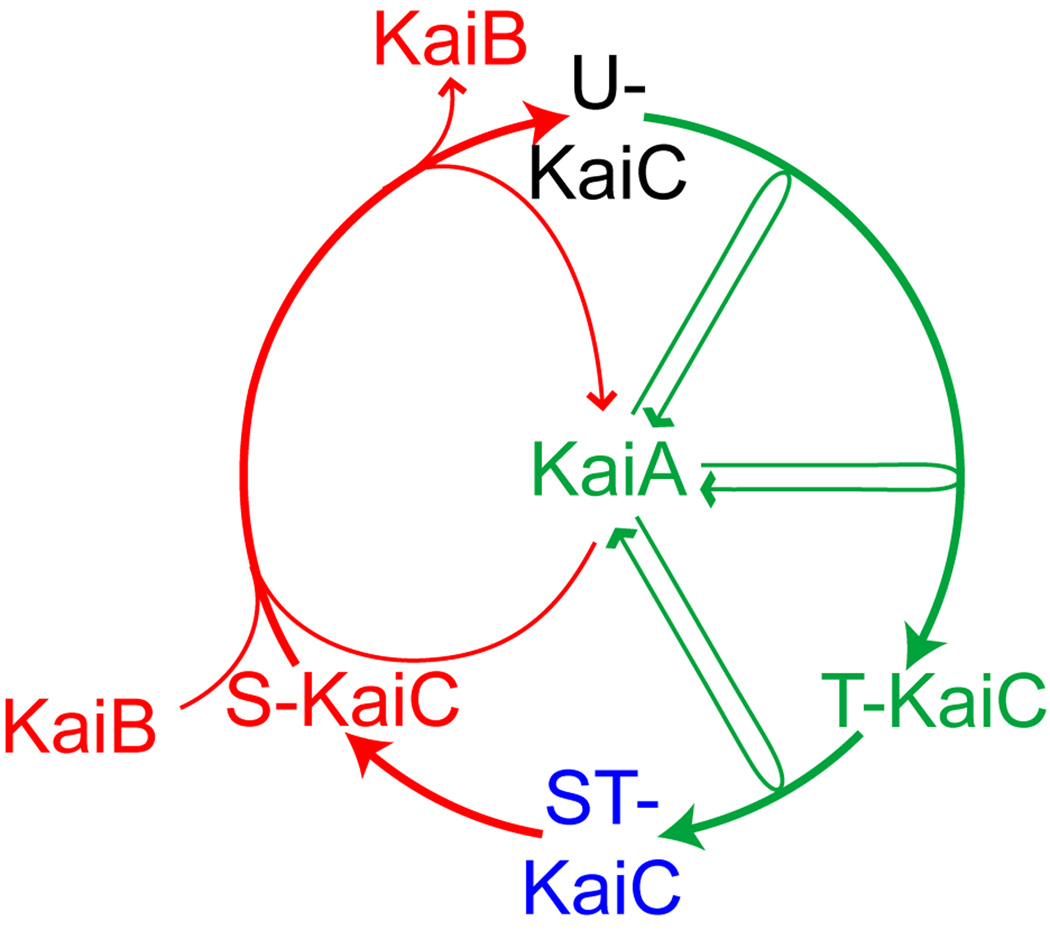
Figure 1. A model of the KaiC phosphorylation rhythm.
During a circadian cycle (represented by a circle), the phosphorylation states of KaiC proceed in an orderly manner. The relative timing of the peak for each phosphoform, based on published data [5,6], is shown by its position on the circle. KaiA stimulates KaiC phosphorylation by repeated association with KaiC. Starting from unphosphorylated KaiC (U); KaiC is first phosphorylated at T432 (T), which is further phosphorylated to the fully phosphorylated form (ST); T432 residue dephosphorylates from ST-KaiC first, resulting in KaiC phosphorylated only at S431 (S). KaiB preferentially binds S-KaiC, which forms a ternary complex with KaiA and, presumably, inactivates it and allows KaiC to return to the unphosphorylated state. The phosphorylation phase is represented in green and the dephosphorylation phase in red.