Figure 3.
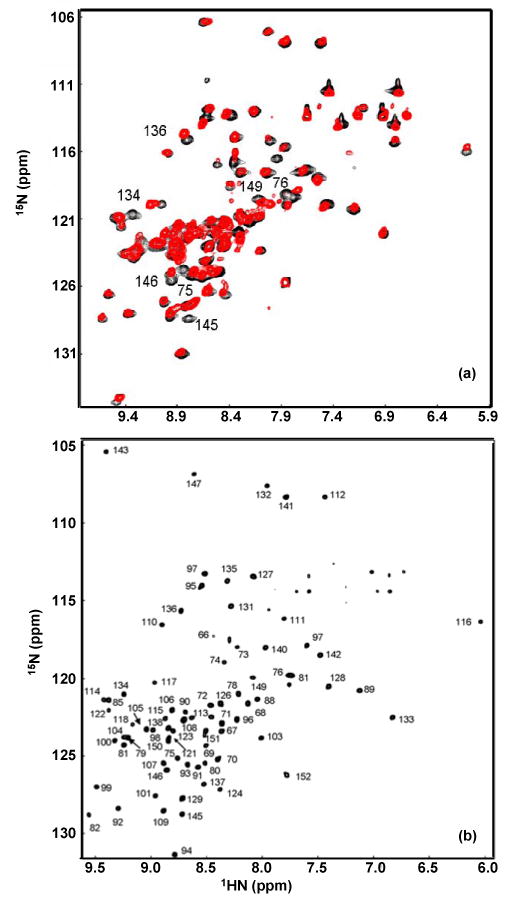
(a). 1H-15N HSQC overlay of 15N-αB10.1 (red) and 15N-N146D-αB10.1 (black). The spectra were acquired on Bruker DMX500 at 22 °C on 0.5 mM samples in 50 mM sodium phosphate buffer (pH 7.5) containing 100 mM Na2SO4. 32 transients per t1 value were acquired. (b). 1H-15N TROSY-HSQC spectrum of [2H, 13C, 15N]-N146D-αB10.1 acquired on Varian Inova 800 on a 1 mM sample. 8 transients per t1 value were acquired. Other conditions are similar to that described in (a). The spectral width in (b) is smaller compared to (a) and so the most downfield peak in (a) corresponding to L143 is aliased in (b). Peaks in (b) are labeled with assignments. Resonances exhibiting chemical shift changes between the wt and the variant spectra are labeled in (a). These resonances belong to residues in β3, β8, and β9 as expected for the topology β3-β9-β8.
