Figure 6.
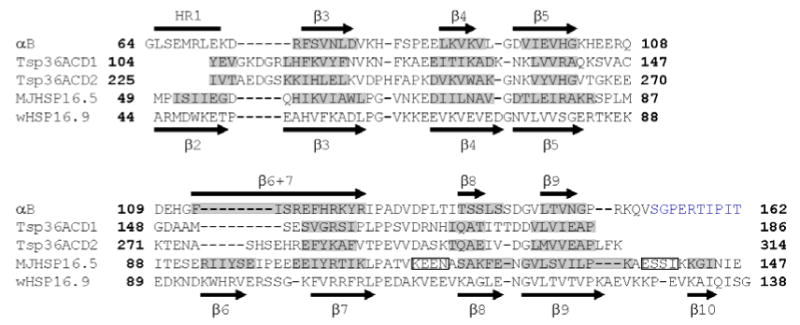
Secondary structures and sequences of the α-crystallin domains from αB, Tsp36, MjHSP16.5, and wHSP16.9 are shown. Tsp36 has two α-crystallin domains, ACD1 and ACD2. The sequence of the α-crystallin domain with an additional 10 C-terminal residues in αB and the corresponding region from wHSP16.9 are shown. The β-strands predicted from solution-state chemical shifts are shown as black arrows above the sequence of αB, those predicted by solid-state data are highlighted in grey along the sequence. The C-terminal residues in αB assigned by solid-state NMR are colored blue. The region corresponding to β2 in other sHSPs is labeled HR1 in αB. Residues M68-K72 have not been assigned by solid-state NMR. Residues G64-E67 and D73 have been assigned and solid-state chemical shifts predict random coil structure similar to solution-state. Residues G64, L65, His83, Phe84, H119, R120, and S139 have not been assigned in solution-state. The secondary structure elements of the α-crystallin domain in wHSP16.9 are shown below the sequence. Segments shaded in grey indicate β-strands in the α-crystallin domains of Tsp36 and MjHSP16.5. Rectangles indicate α-helices in MjHSP16.5.
