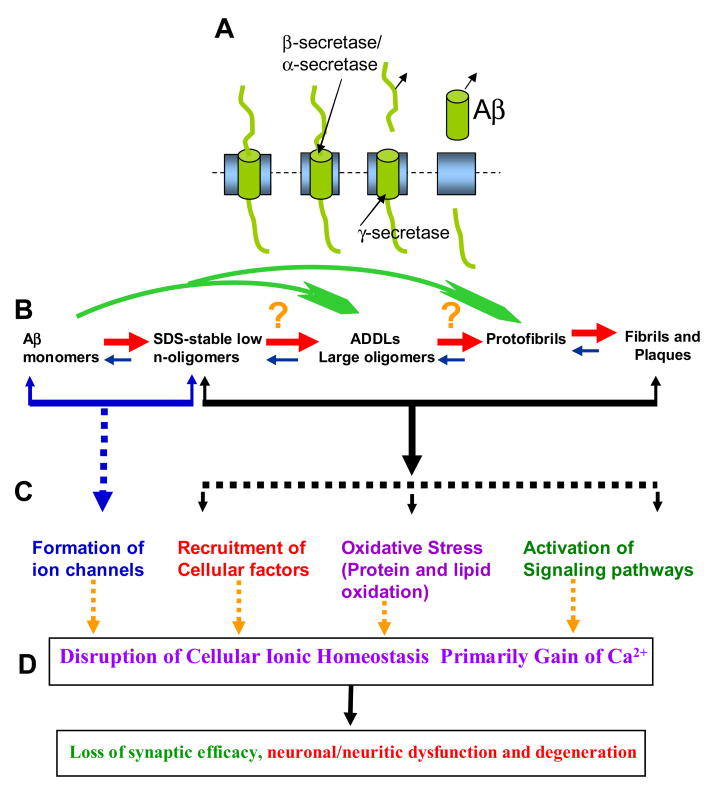
Figure 1.
Ion channel hypothesis for AD pathology. A. Aβ, a 39–43 AA long peptide is cleaved from the single transmembrane spanning precursor, called amyloid precursor protein (APP). B. Aβ undergoes multi-step oligomerization, ranging from the formation of small oligomers to large oligomers (commonly referred to as the ADDLs) to protofibrils to fibrils and plaques. In normal physiological conditions, most of the secreted amyloids remain monomers or small oligomers. C. Several mechanisms for neurotoxic activity of Aβ have been proposed, such as activation of a signaling pathway by extracellular aggregates (fibrils, plagues), induction of oxidative stress due to protein aggregates, or recruitment of factors by intracellular aggregates. Large oligomers (or the ADDLs) may be toxic by non-selectively disrupting cell membrane viability, like a hammer hitting a leaf). Small oligomers (mono- and dimers) fold into the membrane forming ion channels. D. A common denominator for AD toxicity is mainly the gain of intracellular calcium, the severity of toxicity depends upon the level of calcium loading as well as the cell’s defense capability. See [4, 14, 26, 47, 48, 50].